Dear All,
Anthony McDonnell and his colleagues at CGD (Center for Global Development) have just released a truly lovely paper on the economics of antibiotic resistance. What makes this one special is that the paper dissects the problem of AMR through an economist’s spectacles in a way that is very accessible to the non-expert. You may have heard of a market failure, but what is it … really? Is it just something that is expensive, or is it more than that? And what is an externality, negative or positive? And why would I care?
Well, this paper answers those questions and more … read it and learn! Here are the links you need:
- The webpage announcing the paper
- The paper itself: “The Economics of Antibiotic Resistance” by Anthony McDonnell, Ranil Dissanayake, Katherine Klemperer, Flavio Toxvaerd, and Michael Sharland
- A blog about the paper
- Post-newsletter addendum: And if you’d prefer, there is now an excellent 34-minute YouTube explainer … check it out as well as the 19 Mar 2024 newsletter entitled “Tragedy Of The (Antibiotic) Commons: A True Market Failure” in which the video is introduced!
Although you might be tempted to jump straight to the paper, you actually should start with the blog. I was delighted to see how it opened, especially as two of my favorite movies/books on the theme of how “Antibiotics would have changed the plot” are quoted:
- “Beth March died of a disease that would have taken 10 days to treat with penicillin; she was just 23.
- Aside: OMG, Little Women! Beth’s death! See the video discussion!
- “A whole section of Anna Karenina is driven by the prolonged illness and death of the 30-something Nikolai Levin, who could have been cured with a course of antibiotics and very little handholding.
- Aside: OK, I don’t have a video on this one … yet. Will make it happen!
- “Entire essays are devoted to the role of rain-induced illness (most likely pneumococcal pneumonia) as a plot device in Jane Austen.”
- Aside: Straight out of Austen’s Sense and Sensibility! See the video discussion!
Movie vignettes aside, the blog then gives us an excellent high-level tour of the key ideas from the paper. The blog and paper use the same headings/subheadings to take us through a series of VERY deep but important demonstrations that the lack of antibiotics is the result of several different types of market failures:
- The economics of antibiotic resistance
- The Common Pool problem (John says: This is an introduction to the ideas of “public goods” and “non-excludability”)
- The economics of antibiotic supply failures
- Public good characteristics of antibiotic innovations
- Market failures in the production of knowledge
- Regulatory failures exacerbate market failures
- The (unpriced) insurance value of antibiotics
- Market failures in access
- The economics of antibiotic demand failures (John says: The ideas of negative and positive externality are very important — do you know what a Pigouvian tax is?)
- External benefits and public good aspects of infection control
- Misallocation and mismatch problems in human use
- Negative externalities: environmental pollution
- Negative externalities: inappropriate use in agriculture
Why does all this matter? Why should you spend time reading this paper? Well, it’s because this is how economists and Ministers of Finance think — and sometimes the ideas are not what you expect! To quote just one example from the paper:
- “Pharmaceutical research is expensive. Kevin Outterson estimates that an incentive for new antibiotics needs to pay around 3.1 billion USD, which could be paid out over a decade at a rate of $310 million a year (Outterson, 2021).
- “This alone does not imply any market failure, as many expensive things are built and sold privately; indeed, this is not even particularly expensive for pharmaceuticals. The global revenue of the pharmaceutical industry was $1,420 billion in 2021 (Mikulic, 2023).
- All of the 200 top selling drugs that year, as tracked by Pharmalive, earn significantly more than the proposed incentive needed for a new antibiotic.
- Indeed, 48 products had greater revenue in 2021 alone than the proposed lifetime incentive for a new antibiotic, and the 200th best-selling product in 2021 (Takhzyro) earnt $870 million—2.8 times the annual revenue it was suggested that a new antibiotic needs (Humphreys, 2022).
- “However, novel antibiotics are underprovided relative to their social value.”
You might think that “expensive” is the issue but in fact it is that last statement about the mismatch with social value that creates the market failure. In support of this idea, the text moves on to discuss the fire extinguisher values (aka, the STEDI values) of antibiotics — merely by existing, by being available to put out the fire of a resistant infection, antibiotics have a social value that goes beyond the life-saving impact for the treated patient. And this, in turn, takes us to discussions that have meaning at an economic level — by keeping all of us healthy, antibiotics enable economies to flourish … and that’s what a Minister of Finance seeks to enable!
The paper also includes some excellent visuals — here are my two favorites. The first shows the problem of antibiotic supply and demand as a visual using the idea of water supply and demand; the second highlights the relative lack of work in this space:

The clever thing here is that supply/demand ideas from classical economic analysis are woven into the graphic without the need for a lot of words.
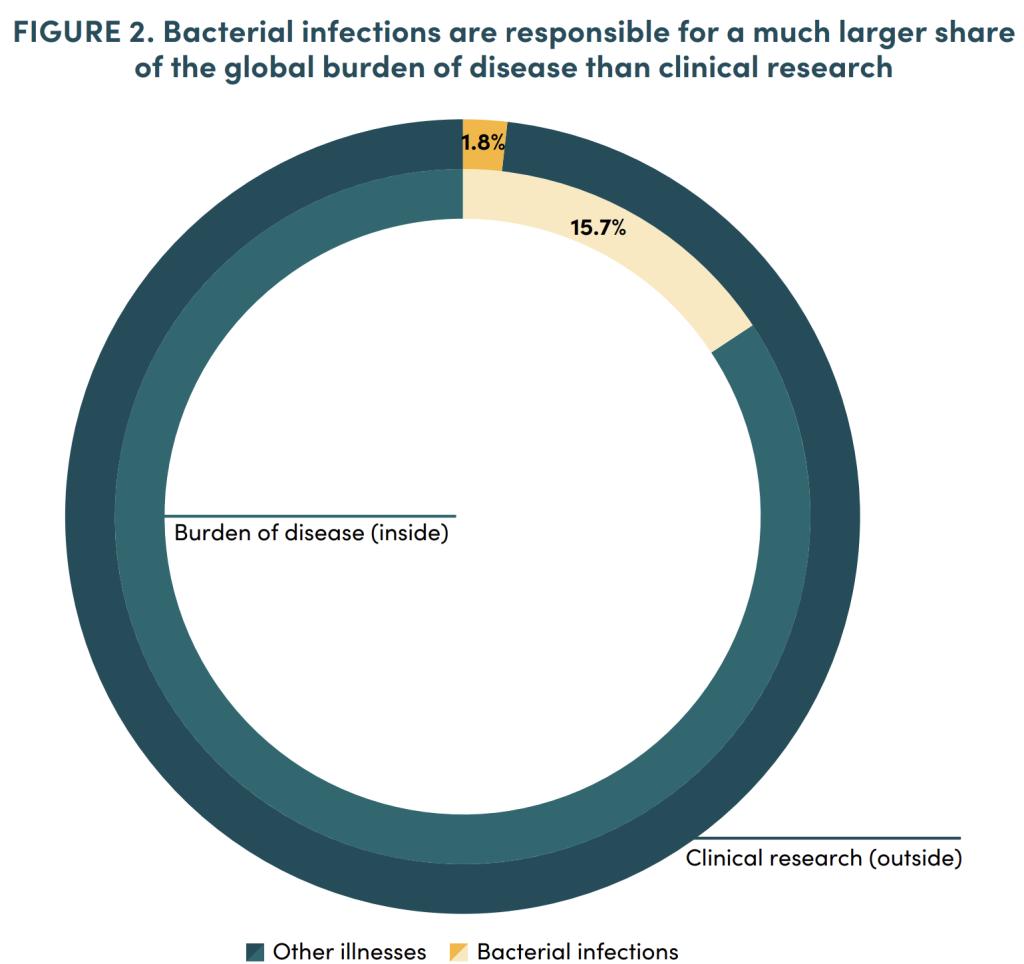
This second graphic uses recent data on the burden of AMR to conclude that “… fewer than 1.8 percent of the 13,605 Phase II and Phase III clinical trials recruiting patients as of August 21, 2023 relate to bacterial infections (as tracked on clinicaltrials.gov), even though 15.7 percent of global deaths are from bacterial infections, of which one-half to two-thirds of which are linked to resistance (Murray et al., 2022)”
And if that weren’t enough, there’s a superb glossary on page 26 summarizing the buzzwords used by economists when talking about market failures and such.
—
Wow! Please read this paper! Although those of us who are techy nerds might wish it would be enough to say “Bad Bugs, No Drugs“, it actually is not enough … that’s probably my central lesson over the past 15 years. We as a community must explain the problem in ways that both punch through the noise of other issues and that resonate with those who hold the political purse strings. This paper is an excellent introduction into that sort of storytelling.
With thanks to Team CGD and all best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- NIAID have a BAA open through 13 Mar 2024 for projects covering vaccines, therapeutics vs. selected pathogens (specific viruses, fungi, and bacteria), and sequencing-based diagnostics. See this newsletter for further details.
- JPIAMR have an AMR Interventions call that is open for pre-applications through 14 Mar 2024. The call covers interventions for both fungi and bacteria. Go here for full details. Note that there is an informational 24 Jan 2024 webinar for applicants.
- ARPA-H have an Open BAA that is accepting applications through 14 March 2024. It is quite wide-ranging in its scope and definitely includes AMR-related projects. See this newsletter for discussion of the BAA and an AMR project that it now supports.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 6-7 Mar 2024 (Basel, Switzerland): The 8th AMR Conference 2024. Go here to register!
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here for the meeting’s general website. You can’t register (yet) for the 2024 event, but save the date!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
Upcoming meetings of interest to the AMR community:
- 27 Feb 2024 (virtual, 8.30a-9.30a EST): GARDP’s “What does the future look like if pull incentives to support antibiotic R&D are insufficient?” webinar. Go here for details.
- 27 Feb 2024 (in person, New York City, 3-6.30p ET): Hosted by the AMR Industry Alliance (AMRIA), “A Call-to-Action in the Fight Against AMR: Priorities for Progress at the 2024 UN High-level Meeting on AMR” is a symposium (3-5.30p) and reception (5.30-6.30p). For additional information on the event, please contact J.Schumacher@AMRIndustryAlliance.org.
- 4-5 Mar 2024 (virtual, noon-4.15p ET on both days): “Assessing the Burden and Potential Strategies to Address Antimicrobial Resistance” is a 2-day workshop sponsored by the National Academies that takes a deep dive into what we do and do not know about the costs (clinical and financial) of AMR. Chaired by Jomana Musmar, the deeply knowledgeable Designated Federal Officer who oversees PACCARB, it is no surprise that the agenda is excellent and includes an all-star cast of speakers. Go here to register.
- 6-7 Mar 2024 (Basel, 6-7 Mar 2024): See Recurring Meetings list, above.
- 17-22 Mar 2024 (Ventura Beach, CA, in person): Gordon Research Conference (GRC) entitled “New Antibacterial Discovery and Development” with a 16-17 Mar 2024 pre-conference Gordon Research Seminar (GRS) for young doctoral and post-doctoral researchers. An intensive residential meeting, GRCs are highly recommended for networking and deep research insights. Apply here for the GRC and here for the GRS.
- 25-26 Mar 2024 (In person, London): “Novel Diagnostics for Infectious Diseases,” a 2-day workshop co-organised and funded by Imperial College London’s Institute of Infection, JPIAMR-funded B2B2B Network, London In Vitro Diagnostics Co-operative, NIHR Imperial Biomedical Research Centre, and DIAMONDS consortium. Go here for details and to register.
- 10-11 Apr 2024 (virtual): Sepsis Alliance AMR Conference, a 2-day conference focused on “practical technologies to manage sepsis and counteract the expanding challenge of antimicrobial resistance.” Go here for details and to register.
- 26 Apr 2024 (Barcelona, Spain): ESCMID workshop entitled “Using Data Science and Machine Learning for Infection Science: A Hands-on Introduction.” Click here to register or here for more details.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- [NEW] 28-29 May 2024 (in person, Uppsala, Sweden): Uppsala Antibiotic Days, a broad-ranging 2-day program hosted by the Uppsala Antibiotic Center. Go here for details and to register.
- 9-13 June 2024 (in person, Ascona, Switzerland): “New Approaches to Combat Antibiotic-Resistant Bacteria, 2nd Edition” is a Sunday-Thursday residential workshop focused on the deep biology of AMR. Sponsored by NCCR AntiResist (a Swiss National Science Foundation consortium), the scientific program has the feel of a Gordon Conference. Space is limited, so you are encouraged to apply promptly — go here for details.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.