Dear All:
Just released today is an RFP (see text just below my signature) to develop a business plan for the design, funding, and implementation of a sustainable clinical trial network for developing new antibacterial agents.
The core of this idea emerged in early 2016 based on debates about the potential value of a network focused on a specific infection (e.g., nosocomial pneumonia) in which a standing protocol randomized subjects to a well-accepted standard comparator (probably a carbapenem) or a rotating series of investigational agents. A group of us summarized the key elements of the idea in this 2016 paper in Clinical Infectious Diseases. Here’s a visual illustration of the concept:
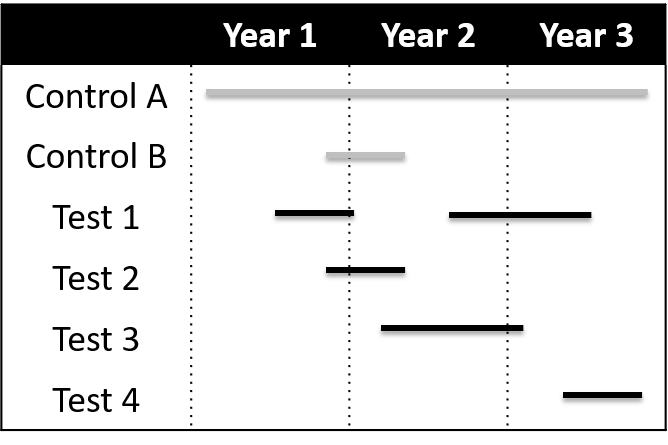
Figure legend from the CID paper: Shown are 3 years in the life of a network. To start the network, a target infection is chosen, a standard protocol is prepared, and a widely registered excellent standard comparator (control A) is selected. After a brief run-in period, test drugs are flexibly added and removed from the network. A constant control arm (control A) is envisioned, but alternative controls can also be used as needed to address issues such as blinding (control B paired with test 2). Multiple agents can be in the network simultaneously. It is also assumed that data on control agents can be shared across drugs.
The goal of the network is to reliably provide sponsors with a data from a properly powered and designed non-inferiority study that would serve as the basis for registration. When combined with implementation by the sponsor of a separate MDR/XDR study, you have the essence of a Tier B trial program.
The network study would be implemented in UDR (Usual Drug Resistance) settings in which a standard comparator such as a carbapenem would be acceptable. Sharing of controls and elimination of study startup delays for each new drug are estimated to reduce cost and time by ~40% for drugs using the network. Continuity for sites and investigators is provided by the ongoing nature of the network.
First discussed in early 2016, it is exciting to see Wellcome taking the next step towards making this concept a reality! Contact details are provided below should you or a colleague be interested in taking up this challenge. Expressions of interest are due by 12 March.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://13.43.35.2/blog/
Wellcome Trust RFP (Request for proposals)
We’re looking to commission an organisation or consortium with understanding of the development and operations of clinical trial networks.
This follows our report on the importance of clinical trial networks for antibiotic development, and how they should be developed.
The purpose of this commission is to develop a business plan for the design, funding and implementation of a sustainable clinical trial network for infectious diseases centred specifically on antibiotic development.
The network should be able to:
- generate comparative data on new agents quickly and efficiently
- evaluate more than one investigational agent at a time using a standardised master protocol for each infection.
Our aim is that the findings from this work will inform the development of a new clinical trials network, helping to reduce costs, get clinical trials up and running more quickly, and lead to shorter late-stage trials.
Supporting documents, including a detailed timeline can be found on our website.
For more information or to express your interest, please email CTNetwork@wellcome.ac.uk. The deadline for expressing interest is Monday 12 March, 23:59 GMT.
Upcoming meetings of interest to the AMR community:
- 11-16 Mar 2018 (Ventura Beach): Gordon Research Conference on Antibacterial Discovery
- 12-13 Mar 2018 (London): BSAC’s Spring Conference
- 19 Mar 2018 (Washington: Duke-Margolis & FDA event: “Utilizing Innovative Statistical Methods and Trial Designs in Rare Disease Settings”
- 20 Mar 2018 (Washington): FDA workshop on complex, innovative trial designs. See also this FR notice.
- 27-29 Mar 2018 (Cardiff, UK): BSAC-sponsored 3-day residential Workshop on Susceptibility Testing
- 16 Apr 2018 (Washington): Duke-Margolis & FDA event: “Evaluating Inclusion and Exclusion Criteria in Clinical Trials”
- 21-24 Apr 2018 (Madrid): ECCMID
- 4-7 Jun 2018 (Boston): BIO (multiple AMR-focused sessions are expected)
- 7-11 Jun 2018 (Atlanta): ASM Microbe
- 22-27 Jul 2018 (Bryant University, Smithfield, RI): Gordon Research Conference on Drug Resistance for Cancer, Infectious Disease and Agriculture
- [UPDATED LINK] 4-7 Sep 2018 ESCMID-ASM Conference (#3) on Drug Development for AMR (Lisbon, Portugal)
- 6-14 Oct 2018 International Course on Antibiotics and Resistance (ICARe, Les Pensières, Annecy, France)