Dear All,
3 Apr 2020 update: Significant additional details on the pilot were shared via a 29 Mar 2020 webinar (link to newsletter) and then a FAQ document (link to newsletter). The game is definitely afoot!
The hoofbeats of the cavalry bringing us the UK NHS antibiotic subscription model are definitely getting louder! As you may know, NHS England is collaborating with NICE on a pilot project under which NHS England will buy two antibiotics on a delinked (volume- and usage-independent) subscription model basis. If you need more background, see this post by the UK government. For supplemental detail, this blog provides links to substantial background context on the rationale for this approach.
As this is exactly the kind of delinked market incentive that we need, it was very exciting to hear concrete plans laid out by the NHS-NICE team at a 25 Nov 2019 webinar. The focus of the webinar was on how the team is using public feedback to adjust the process to ensure transparency and fairness (go here for the slides; the team can be reached at ABpaymentmodels@nice.nhs.uk).
These two slides give the key points of process and timelines:

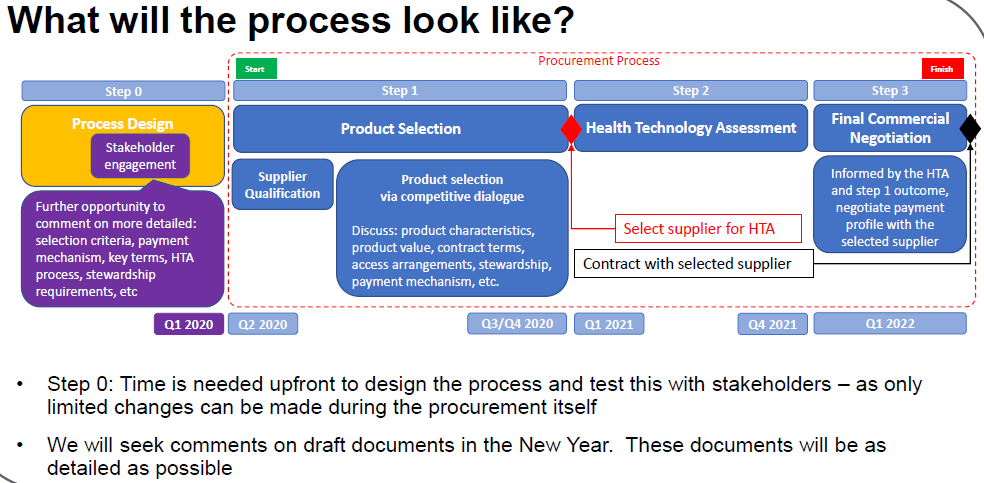
Key points are that the selection of products will be via a public procurement process that will run during 2020 with a final contract anticipated for 2022. The procurement process will follow the UK’s PCR2015 (Public Contracts Regulation 2015) and will use a Competitive Dialogue-based approach (go here for more details) that permits the NHS to flexibly update the requirements based on dialogue with applicants.
Also of interest is the way that the project team seeks to avoid foot faults and legal challenges via a transparent approach to external advice. Here we have two more relevant slides in which we learn about a Stakeholder Forum, a Project Advisory Group, and an Expert Panel:

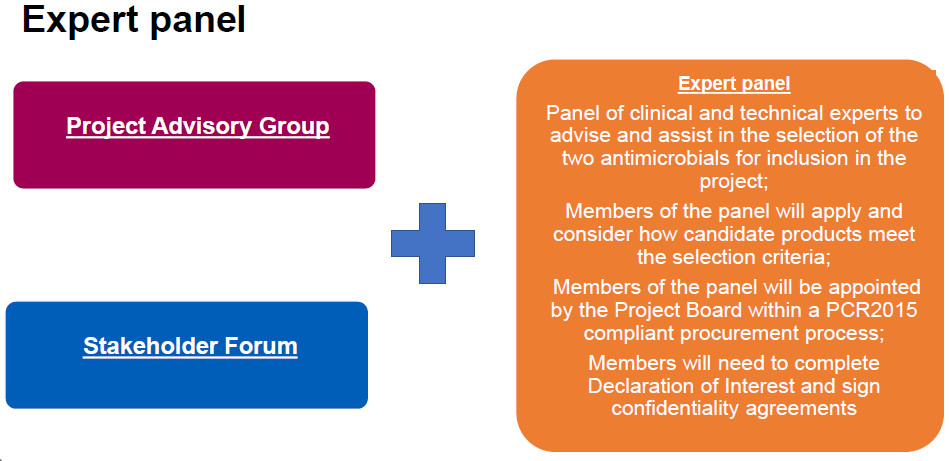
The steady progress on this project is very exciting! In particular, I really applaud the team’s careful approach to this first experiment. The world will be watching: an error-free pilot run with a fair and even hand should be the goal and I think the team is well on its way to achieving this!
Best wishes and Happy Thanksgiving to all who celebrate that holiday! –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/
Upcoming meetings of interest to the AMR community:
- 5 Dec 2019 (Monthey, Switzerland): The BioArk technology park is holding a one-day workshop on AMR. Entitled “The Ark Life Sciences Series #1”, you can get more details here.
- 16-18 Dec 2019 (Bangkok, Thailand): 3rd International Symposium on Alternatives to Antibiotics in Animal Production. Go here for details: https://web.archive.org/web/20170227033533/https://www.ars.usda.gov/alternativestoantibiotics/
- 16 Jan 2020 (Washington, DC): Duke-Margolis meeting entitled (approximately) “improving Payment Policies for Antibiotics.” This meeting will run 10:30am – 4:30pm ET. Go here to register.
- 20 Feb 2020 (London, UK): Westminster Health Forum conference entitled “Antimicrobial resistance – coordinating a global response and progress on the UK strategy.” Go here for details.
- 26-27 Feb 2020 (Washington, DC): US PACCARB public meeting. Go here for details.
- 1-6 Mar 2020 (Il Ciocco, Tuscany, Italy): Gordon Research Conference (GRC) on Antibacterial Discovery and Development: “Now is the time to re-boot antibiotic R&D before it’s too little, too late.” Go here for details.
- 12-13 Mar 2020 (Basel): BEAM-, Novo REPAIR-, CARB-X-, DZIF-, ND4BB-, ENABLE-supported (among a long list!) Conference on Novel Antimicrobials and AMR Diagnostics. Details are here, poster deadline is 12 Dec 2019.
- 16-17 Mar 2020 (London): BSAC Spring Conference entitled: “Bridging the gap between science, policy and effective antimicrobial use.” Go here for details.
- 18-21 Apr 2020 (Paris): Annual ECCMID meeting (#30)
- 25-30 May 2020 (Rotterdam), Annual ESPID meeting (European Society for Pediatric ID, #38)
- 27-28 Jun 2020 (Bryant University, Rhode Island): Drug Resistance Gordon Research Seminar entitled “Mechanisms and Approaches to Overcoming Drug Resistance in Cancer, Infectious Disease and Agriculture” for graduate students and postdoctoral scientists. Go here for details … this immediately precedes the GRC listed just next
- 28 Jun-3 Jul 2020 (Bryant University, Rhode Island): Gordon Research Conference (GRC) entitled “Strategies to Disrupt Drug Resistance in Infectious Disease, Cancer and Agriculture.” Go here for details.
- 1-4 Sep 2020 (Dublin): Annual ASM-ESCMID Conference on Antibiotic Development #5! Mark your calendar now and go here for details.
- 9-10 Sep 2020 (Washington, DC): US PACCARB public meeting. Go here for details.
- 22-25 Sep 2020 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 17-25 Oct 2020 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics and resistance. The course is very intense, very detailed, and gets rave reviews. The date is set for 2020 and the program will ultimately appear here. Registration is limited to 40 students and opens 15 Mar 2020.
- 10-13 Apr 2021 (Vienna): Annual ECCMID meeting (#31)