Dear All (Wonkish alert! Coffee-up!),
Today we have something special: A deep-dive into the future trajectory of AMR-related work developed by Wellcome Trust. The newly released report is here and there is a webinar discussing the report on Wednesday of this week (1500-16:00 GMT on 18 Nov 2020, go here to register).
The back story on this report begins in 2016 when a UN High-Level Meeting on AMR came together as a response to many years of awareness-raising. This was only the fourth time in the history of the UN that a health topic was discussed at the General Assembly and it spurred global political momentum on the whole AMR issue. Deep background if you are interested: to get a flavor for past efforts, review this newsletter focused on ways to tell the AMR story and this newsletter focused on financing the needed work.
Beginning in 2019, Wellcome Trust set out to review progress since 2016. When COVID hit earlier this year, the review was expanded to include the impact of COVID both on AMR and on general awareness of the need for preparedness. Not surprisingly, there is both good news and bad news:
- Successes
- AMR has achieved prominence on the global political agenda
- The AMR community is a broad, multi-sectoral coalition of actors aware of, and willing to tackle, AMR
- The early-stage and translational research environment is robustly funded
- Critical gaps
- Activity has not always translated into meaningful action
- Prioritization is increasingly emerging as a gap
- The AMR agenda was at risk of losing momentum pre-COVID-19 – making it important to capture new momentum in global health with a clear post-COVID-19 AMR narrative
The three successes will be familiar to regular readers of this newsletter, as will the first gap. But, the gaps around prioritization and momentum identified by the report are the real novelty of the report. In brief, there are always many big issues competing for attention and the AMR community has been criticized at times for being a “talking shop” in which little happens. As a corrective, the report is saying that the AMR community must maintain momentum by applying a disciplined and focused approach to its work over the next several decades.
You should read at least the Executive Summary for yourself, but the essence is that the report sketches a potential ‘critical path’ to impact by seeing needed work in two phases:
- 2020–30: (i) Mitigation of the risk of resistance and its consequences and (ii) Expansion of the evidence base to address gaps that remain as barriers to action.
- Beyond 2030: Building on an established infrastructure to control resistance and its consequences, work to limit further resistance development by maintaining and scaling best practices for prevention (clean food, clean water, vaccines, etc.)
The really fascinating aspect of this paper is its clear-eyed and provocative discussion of priorities for the AMR field. The analysis is summarized by the categorization of 13 areas of possible focus across a 3×3 grid that ranks impact vs. feasibility (Exhibit 5, page 33):
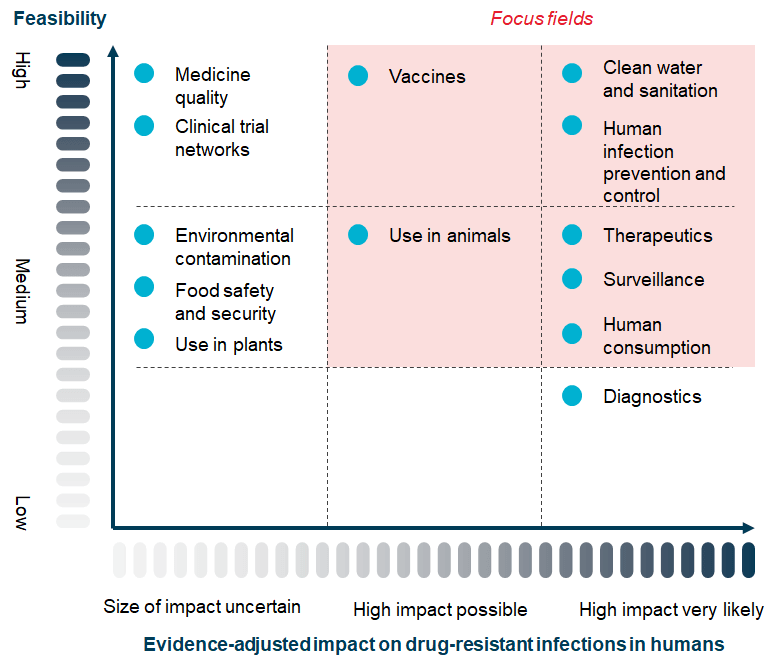
Look closely at the grid: Feasibility on the y-axis, impact on the x-axis, 13 topics placed in the resulting grid, and (finally) pink shading that highlights the seven focus areas recommended by the report as highest priority. Each of the 13 topics has a detailed discussion in the report and to whet your appetite to read the full report, let’s consider just one that falls outside the highlighted pink focus area: Diagnostics.
As you can see, Diagnostics are scored as “High Impact Very Likely” but “Low Feasibility.” Hence the report suggests that it should not be ignored but that (importantly) Diagnostics should not be a make-or-break highest priority area. Why, you ask? Well, the extended notes conclude that “… both the therapeutics and the diagnostics ecosystems face similar market failures and lack of investment due to low expectations on returns. However, diagnostics suffer from additional behavioural and structural barriers to uptake, making it more difficult to resolve compared to the already complex therapeutics environment.”
The other possible focus areas are likewise analyzed at length (see below my signature for a brief summary of the key points regarding each). Over and over, the point is that while all 13 areas have value, differences in feasibility and likely impact should not be ignored!
Putting it all together, the core message of the report is that attempting to do everything will lead to getting nothing done. Rather, we need to take stepwise-actions that are relevant, focused and feasible, and that build towards the long-term goal of controlling AMR.
And this is where COVID-19 enters into the discussion. There has been a lot of discussion of the impact of COVID-19 on AMR, often focused on emerging resistance. That’s of interest, but to my eye the bigger issue is how to use the awareness created by COVID-19 to drive work in AMR. Wellcome’s report is distinctive to my eye in that it makes the case that there is a decision-point facing the AMR community: we can reinvigorate the AMR agenda either by trying to stay focused on it as a unique problem or integrate a focused AMR agenda into the broader health security agenda. The report clearly favors the latter approach … and I agree entirely.
Wow! Great stuff! Many thanks to the team at Wellcome for this insightful and provocative commentary. I am sure we’re going to have some spirited debates as a result!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Narrative summary of the 13 areas for possible focus
- High feasibility; high impact very likely
- Human Infection Prevention and Control (IPC): There is some need for additional evidence on best interventions in High/Low Income Countries, but overall IPC interventions are cost-effective and high impact.
- Clean Water and Sanitation: WASH (Water, Sanitation, and Hygiene) are easily shown to be low-cost and high-impact.
- Medium feasibility; high impact very likely
- Therapeutics: This one is divided by the report into R&D and Development. On R&D, we have “… development of new therapeutics is widely considered to be a short- and mid-term priority for the AMR response.” And on Development, we have a good summary of the Push/Pull problem and how it leads to our thin pipeline. Absolutely agreed on both counts.
- Surveillance: Adequate surveillance is easily seen as both feasible and high impact. Good work is underway (e.g., WHO’s GLASS program).
- Human Consumption of Antibiotics: This one seems straightforward — good stewardship along with good health infrastructure are challenging but have a strong impact when implemented.
- High feasibility; high impact possible
- Vaccines: The analysis covers both human and animal vaccines noting the obvious potential for impact with both, albeit with different sorts of implementation challenges.
- Medium feasibility; high impact possible
- Use in Animals: Given the high volumes of antimicrobials used in animal health, successful interventions can have a substantial impact. Good examples exist and are being implemented.
- Low feasibility and/or uncertain impact
- Medicine Quality: This is good example of something that is obviously important but just hard to manage as the required fixes include evidence generation, regulation reform, and systems engineering to detect low-quality medicines.
- Clinical Trial Networks: This is one that I’ve long wished for but I also see why it is a tough sell — it doesn’t directly drive innovation and is unlikely to have an impact without an overall change in the economics of antibiotics.
- Environmental Contamination: The challenge here is one of information: It’s very hard to show causality. Absence of evidence is not evidence of absence, but…
- Food Safety and Security: The limitation here seems to focus on the ability of national governments to create and enforce effective regulatory actions to protect citizens from foodborne illnesses.
- Use in Plants: The issues here are (i) Awareness of the risk (AMR in plants!?) and (ii) lack of data.
- Diagnostics: As discussed in the body of the newsletter, market failure challenges plus structural barriers to implementation create challenges.
Current funding opportunities (most current list is here):
- The National Institute of Allergy and Infectious Diseases (NIAID) Applicant Assistance Program (AAP) opens on October 22, 2020. This program provides no cost support for companies planning to apply for a Phase II, Fast Track, or Direct-to-Phase II SBIR or STTR Award. Go here for details.
- Novo REPAIR Impact Fund closed its most recent round on 31 Jul 2020. Go here for current details.
- 2020 funding rounds for CARB-X have not been announced.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes funders and projects by geography, stage, and more.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- ARLG (Antibiotic Resistance Leadership Group, link) is currently open for applications for its 2-year ARLG Fellowship program. The application deadline is 1 Dec 2020; full details are here.
- Finally, you might also be interested in the most current lists of R&D incentives (link) and priority pathogens (link)
Upcoming meetings of interest to the AMR community (most current list is here):
- 16 Nov 2020 (online, 9.30a-4.00p EST): FDA workshop entitled “Potential Approach for Ranking of Antimicrobial Drugs According to Their Importance in Human Medicine: A Risk Management Tool for Antimicrobial New Animal Drugs.” Go here for the FR notice and here for extended details, including registration.
- 17 Nov 2020 (online, 17:00-18:30 CET): GARDP-sponsored webinar entitled “Discovery of new antibacterials using artificial intelligence (computational chemoinformatics)” moderated by Laura Piddock. Go here to register.
- [NEW] 18 Nov 2020 (online, 15:00-16:00 GMT, London): Wellcome Trust-sponsored webinar entitled “The Global Response to AMR.” This will be the launch of a report by Wellcome on progress and gaps in addressing AMR since the 2016 UN High-Level AMR Meeting. Go here to register.
- 18 Nov 2020 (online, 14:30-15:30 CET): GARDP-sponsored webinar entitled “Drugs don’t work: What can be done to fight the threat of antibiotic resistance for effective cancer treatments?” moderated by Ingrid Stenstadvold Ross (CEO, Norwegian Cancer Society). Go here to register.
- 18-24 Nov 2020 (everywhere): World Antimicrobial Awareness Week. For resources, go here for WHO’s home page for the week. The focus will be on two messages: “Antimicrobials: handle with care” and “United to preserve antimicrobials.”
- 19 Nov 2020 (online, 9-10.30am EST) webinar chaired by Jeremy Knox entitled “Responding to difficult-to-treat infections: Role and responsibilities of governments, researchers, clinicians, industry and patients”, the final webinar in a 4-part series sponsored by Wellcome Trust entitled “AMR in the Light of COVID-19 Webinar Series; From hypothetical to reality: How COVID-19 foretells a world without antibiotics.” Go here to register.
- 20-22 Nov 2020 (online, 13:00-18:00 GMT: Global AMR Youth Summit sponsored by the World Health Students’ Alliance (WHSA). Go here to register.
- 20 Nov 2020 (online, 10:30a-noon CET): GARDP-sponsored webinar entitled “Saving childrens’ lives – treating neonatal sepsis” moderated by Peter Beyer (Senior Advisor on AMR, WHO). Go here to register.
- [NEW] 2 Dec 2020 (online, 3 sessions spanning 5am-5.15p GMT): Global AMR Hub-sponsored conference entitled “Translating AMR R&D mapping into policy and action.” Go here for the full program
- 5-7.15a GMT: “Identifying research gaps to address antimicrobial resistance relevant to the Asia Pacific Region”
- Noon-2.15p GMT: Filling AMR R&D gaps in animal health at country, regional and global level
- 3p-5.15p GMT: Working together to fill AMR R&D gaps – collaboration and partnerships
- [NEW] 3 Dec 2020 (online, 9-10:30a CET; 5-6:30p KST): Multi-sponsored webinar entitled “Evidence to Action – Advancing the Antimicrobial Resistance agenda during a pandemic.” Go here to register. Co-hosted by the International Vaccine Institute (IVI), the International Centre for Antimicrobial Resistance Solutions (ICARS), and the Embassy of Denmark in Korea, this webinar includes Dame Sally, Hanan Balkhy (WHO), and the Minster of Health from both South Korea and Denmark!
- 26-28 Jan 2021 (online, runs ~7.30a-5.00p Central each day): 4th Annual Texas Medical Center Antimicrobial Resistance and Stewardship Conference. Sponsored by McGovern Medical School, ARLG, and the Gulf Coast Consortia, the agenda includes both poster sessions and keynotes. The call for abstracts closes 18 Dec 2020. Go here for more details.
- 10-12 Mar 2021 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register; abstract deadline is 15 Nov 2020.
- 9-12 Jul 2021 (Vienna): Annual ECCMID meeting (#31)
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 3-7 Jun 2021 (Anaheim), ASM Microbe 2021. Go here for details.
- 27 Jun-2 Jul 2021 (Ventura, CA): Gordon Research Conference entitled “Antimicrobial Peptides”. Go here for details, go here for the linked 26-27 Jun Gordon Research Seminar that precedes it.
- 5-21 Aug 2021 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- [Webinar streaming link posted] 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students. Bonus feature: For obvious reasons, the course didn’t happen in 2020! But as a celebration of the course’s 5th year, a webinar version was held on 29 Oct 2020: go here to stream it.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.