Dear All,
WHO have today updated their 2019 clinical/preclinical pipeline review by releasing their 2020 analyses of both the clinical and pre-clinical antibacterial product pipelines (the new 2020 report, the press release).
Their 2020 review of antibacterial products in Phase 1 and beyond covers both traditional (n = 43) and non-traditional products (n = 27) for WHO priority pathogens, M. tuberculosis, and C. difficile. In addition, (i) vaccines are covered for the first time and (ii) clinical utility of Phase 3 products is estimated in expanded commentaries.
While it is great to see continued effort by the R&D community, WHO wastes no time in saying that those 70 products in the clinic aren’t enough: “Overall, the clinical pipeline and recently approved antibiotics are insufficient to tackle the challenge of increasing emergence and spread of antimicrobial resistance.” In visual terms:
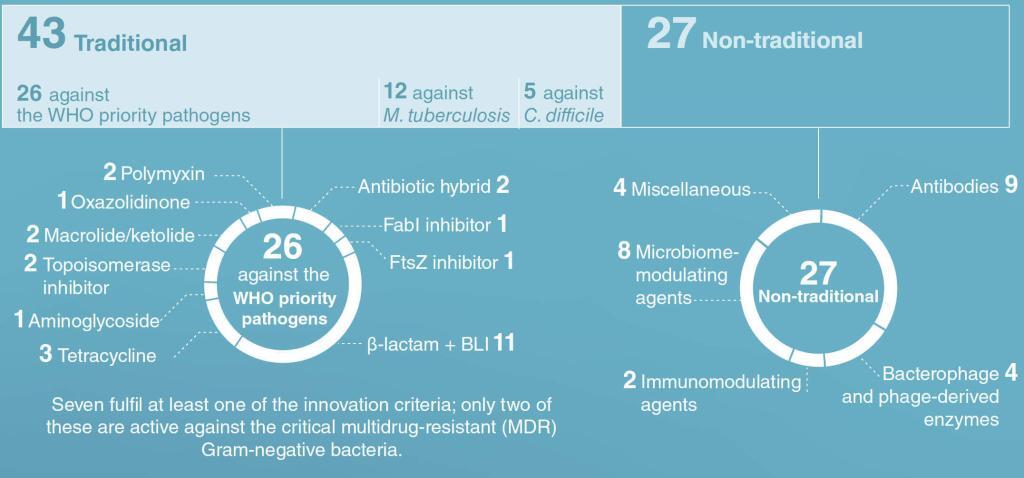
As WHO says, “With some exceptions, the newly approved agents have limited clinical benefit over existing treatment, as over 80% (9/11) are from existing classes where resistance mechanisms are well established and rapid emergence of resistance is foreseen.”
—
That’s the bad news. But you have to remember that true innovation is a long, slow process: drug R&D takes 10-20 years to come to fruition. The push to support really creative antibiotic R&D that started in the mid-20-teens with ND4BB (TRANSLOCATION, ENABLE, etc.), CARB-X (more than 80 funded projects!), JPIAMR (28 collaborating nations), the Novo REPAIR Impact Fund, and more (see the AMR Global Hub’s R&D Dashboard) is just now leading to compelling innovation in the preclinical pipeline.
It is thus encouraging that WHO’s updated 2020 preclinical pipeline review finds that there are 292 preclinical projects targeting WHO priority pathogens, M. tuberculosis, and C. difficile. While most of these projects will fail (that’s the nature of R&D!), you miss 100% of the shots you don’t take, and it is these products that are our future. Thank you, Team WHO, for making an effort to review this rapidly changing segment of the pipeline!
—
Digging deeper: As you review these reports, please also (re)review the excellent clinical pipeline analyses released in March 2021 by the Pew Charitable Trusts. Overall, the Pew and WHO reports have similar findings; here are the links you need for Pew’s reports:
- 10 Mar 2021 newsletter: Overview of Pew Trusts’ analyses, including their marvelous animated pipeline evolution graphic
- 15 Mar 2021 newsletter: Pew Trusts’ infographic of using reimbursement reform to bridge the (Financial) Valley of Death
- 17 Mar 2021 newsletter: Discussion of “Where’s the Innovation?” and of India’s Priority Pathogen List
Also of note if you are doing a deep dive is the excellent 2019 review by Theuretzbacher et al. of the global preclinical pipeline (24 Nov 2019 newsletter).
—
As additional commentary on all of this, WHO’s Peter Beyer and I recently had a chance to chat about the pipeline review and other topics. For those of you who don’t know him, Peter has a PhD in environmental law, became fascinated by the problem of antibiotics, and wound up working at WHO. In his role at WHO as “Senior Advisor, Public Health, Innovation and Intellectual Property,” Peter has been a key driver of the pipeline reviews, GARDP, and much more. Please check out our 40-minute fireside chat!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- Novo REPAIR Impact Fund has an open global call that ends on 7 May 2021. Go here for current details.
- NIAID’s 2021 Broad Agency Announcement for product development is entitled “Development of Medical Countermeasures for Biothreat Agents, Antimicrobial-Resistant Infections an Emerging Infectious Diseases” and is now live with a 24 May 2021 deadline. Research areas include Vaccines, Therapeutics, and Sequencing-Based Diagnostics.
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 20-22 April 2021 (online, 1-5p CET): JPIAMR-sponsored workshop entitled “Feeding the Antimicrobial Therapeutics Pipeline.” JPIAMR, the collaborative global effort of 28 countries (much of the EU and also Argentina, Canada, Egypt, Israel, Japan, Korea, South Africa, and Turkey), is organizing a mixture of keynote talks, abstract presentations, and discussion panels designed to encourage collaborative antibiotic R&D. Go here for more details and registration. Abstract deadline is March 17th 2021; no previous JPIAMR funding required.
- 21-22 April 2021 (online, 9a-5p UK): 2021 BioInfect Conference sponsored by BioNow, the Northern England business support group. Go here to register.
- 23 Apr 2021 (online, 9a-5p EST): FDA-NIAID-CDC workshop entitled “Development Considerations of Antimicrobial Drugs for the Treatment of Gonorrhea.” The workshop’s webpage is here; go here to register.
- 29 April 2021 (online, 5-6.30p CEST): GARDP-sponsored webinar entitled “AMR R&D efforts in the CMC and formulation arena: Do it right the first time!” Go here to register.
- 10-12 May 2021 (virtual): UK-focused Virtual AMR Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free 3-day virtual event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. It will be followed (COVID-willing!) by a face-to-face mission scheduled for 11-15 Oct 2021. Go here for more details.
- [NEW] 13 May 2021 (virtual, 9.30-11.00a EST): CDC-sponsored webinar entitled “AMR in a Changed World: Building Resilient Systems for Today and Tomorrow.” Moderated by CDC’s Michael Craig, an international panel will discuss “where we go from here to address AMR after the COVID-19 pandemic.” Go here to register.
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 24-29 May 2021 (online and in Geneva): ESPID 2021, the 39th Annual Meeting of the European Society for Paediatric Infectious Diseases. Save-the-date announcement is here, details to follow.
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 20-24 Jun 2021 (virtual, various times): World Microbe Forum sponsored by the American Society for Microbiology (ASM) and the Federation of European Microbiological Societies (FEMS). Go here for more details and to register.
- 27 Jun-2 Jul 2021 (Ventura, CA): Gordon Research Conference entitled “Antimicrobial Peptides”. Go here for details, go here for the linked 26-27 Jun Gordon Research Seminar that precedes it.
- 9-12 Jul 2021 (virtual): Annual ECCMID meeting (#31)
- [NEW] 26 Jul-30 Jul 2021 (online): Small World Initiative Instructor Training Workshop – training for undergraduate professors in the wet lab techniques, parallel curricula, & pedagogical instruction to engage students in the hunt to find new antibiotic-producing soil microbes. Go here to register.
- 14-29 Aug 2021 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 11-15 Oct 2021 (physical, somewhere in the UK): UK-focused Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. Go here for more details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students. Bonus feature: For obvious reasons, the course didn’t happen in 2020! But as a celebration of the course’s 5th year, a webinar version was held on 29 Oct 2020: go here to stream it.
- 25-28 Oct 2021 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.