Dear All,
Three global conversations to put on your listen-to-this list! One is this coming week, one is during ECCMID, and one is a video you can listen to now!
First up and happening in just a few days on Thursday 29 Apr (10a-6p EST), please mark your calendar for a UN General Assembly High-Level interactive dialogue on AMR. The available details are somewhat scant, but per the concept note there will be 4 panels that cover these topics:
- AMR in the context of COVID-19 (10.30-11.45a EST)
- Overview of global progress on AMR and vision of the Global Leaders Group (11.45a-1p EST)
- Tackling AMR at country level (3p-4.15p EST)
- Ensuring sufficient and sustainable AMR financing (4.45-5.30p EST)
The session seeks to raise awareness about AMR. Expected to speak are the co-chairs of the Global Leaders Group on AMR (the prime ministers of Barbados and Bangladesh) along with several ministers. As a reminder (see also this 28 Nov 2020 newsletter), the GLG was created in response to the 2019 “No Time to Wait: Securing the Future from Drug-Resistant Infections” report to the UN Secretary-General (UNSG) from the Interagency Coordination Group (IACG) on Antimicrobial Resistance.
Per their Terms of Reference, the GLG has an advisory and advocacy role to address the global AMR crisis by maintaining high levels of global, political and advocacy momentum — and they are clearly achieving this with this high-level dialogue! There’s not a specific way to register but I’m told the High-Level Dialogue will be live-streamed on UN TV. So, mark your calendar and plan to listen in!
Follow-up note: The session is available for streaming on YouTube.
—
Finally, during last week’s EU JPIAMR therapeutics workshop I had the opportunity to chair a 5-person panel entitled “Choosing wisely: Is your product worth developing?” The recording of the video from that conversation with Kevin Outterson (CARB-X), Christine Ardal (DRIVE-AB and EU-JAMRAI), Marco Cavaleri (EMA), Laura Piddock (GARDP), and Camilla Petrycer Hansen (Novo REPAIR), is not yet available. But, you can get a preview of some of the discussion by listening to my recent fireside chat (see below) with Christine Ardal.
The core goal for the panel discussion was to introduce perspectives on how new antibiotics will be evaluated in the future. The idea is that product properties such as the following from the UK’s pilot project are going to be used to rank new products:
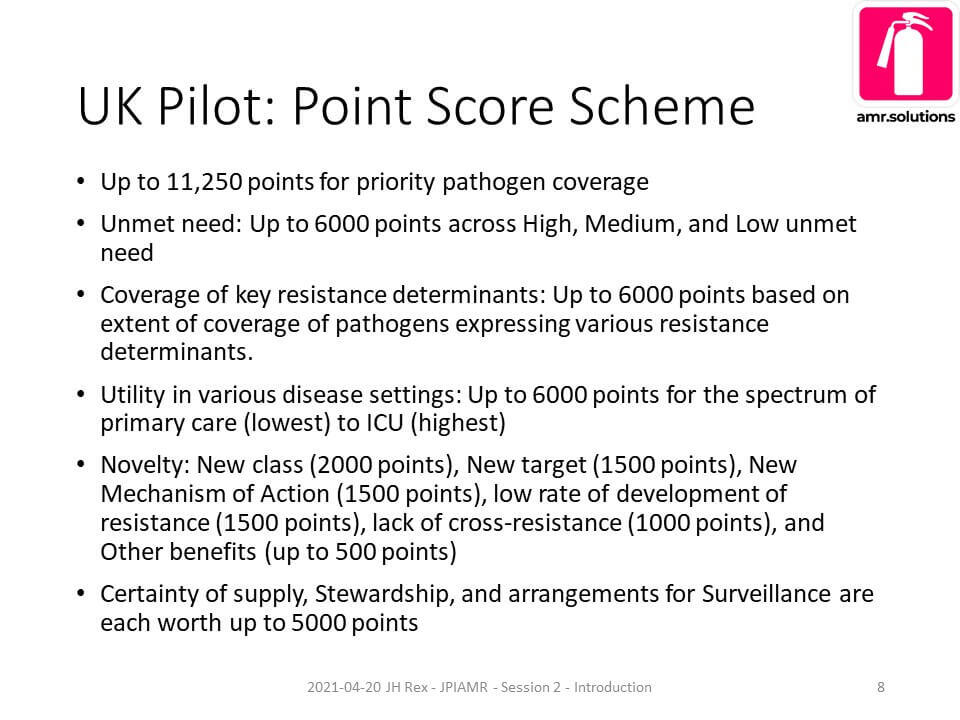
The point (pun intended!) should be self-evident: Possible core scientific features can be known when you start your project! Choose wisely! Aside: you can get this slide from the PowerPoint deck I used to introduce the panel discussion; see also the wonkish details at the end of this 29 Mar 2020 newsletter.
As an interesting parallel build on this theme, the recent EU-JAMRAI policy brief entitled “Incentivizing Antibiotic Access and Innovation” reported outcomes from a survey of views on incentives from 13 countries (10 in the EU plus Canada, Japan, and South Africa). There was support for the idea of incentives from 11 of the 13 countries (hooray!), but the big concern was that perennial theme of “I want to see data on how the drug performs in infections due to resistant bacteria.” Stated differently, this is the non-inferiority/superiority problem about which there is both a 19 Sep 2020 newsletter and a YouTube video.
1 Nov 2021 addendum: Relevant to this theme, please also see the 20 Aug 2021 newslettter entitled “‘Astonishing Mismatch’: Market Potential Of AMR Tools Vs. Patient Needs.” This newsletter discusses a report from the Global AMR R&D Hub that (i) documents (again) the value of new antibiotics and (ii) also provides a fascinating discussion of the very different challenge for diagnostics.
Christine Ardal and I recently had an instructive Fireside Chat about this (and related) themes that I’d encourage you to view. If you don’t have time for the full conversation, please jump to 11:18 to hear the discussion of EU JAMRAI’s findings about product characteristics that will earn substantial delinked reimbursements. My takeaway is that we (the R&D community) need to do a better job of explaining what is and is not possible … but we also need to do what we can to generate at least some data:
Aside: If the issues around generating data in infections due to resistant bacteria are new to you, please do read the newsletter and watch the superiority/non-inferiority video to learn more about this important problem. In brief, it is easy to wish for data from settings in which available drugs are failing but nobody wants to be the patient who receives ineffective therapy!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- Novo REPAIR Impact Fund has an open global call that ends on 7 May 2021. Go here for current details.
- NIAID’s 2021 Broad Agency Announcement for product development is entitled “Development of Medical Countermeasures for Biothreat Agents, Antimicrobial-Resistant Infections an Emerging Infectious Diseases” and is now live with a 24 May 2021 deadline. Research areas include Vaccines, Therapeutics, and Sequencing-Based Diagnostics.
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- [NEW] 27-28 April 2021 (online, 3-7p CET): “EXPERT WORKSHOP: Advancing towards a standardized murine model to evaluate treatments for AMR lung infections” sponsored by the IMI Combine project from within the overall IMI-AMR Accelerator project. Go here to register.
- 29 April 2021 (online, 5-6.30p CEST): GARDP-sponsored webinar entitled “AMR R&D efforts in the CMC and formulation arena: Do it right the first time!” Go here to register.
- [NEW] 29 Apr (10a-6p EST, virtual), UN General Assembly High-Level interactive dialogue on AMR. The event will be live-streamed on UN TV.
- 10-12 May 2021 (virtual): UK-focused Virtual AMR Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free 3-day virtual event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. It will be followed (COVID-willing!) by a face-to-face mission scheduled for 11-15 Oct 2021. Go here for more details.
- [NEW] 12 May 2021 (virtual, 11a-12.30p EST): Duke-Margolis-sponsored webinar entitled “Combating Rising Antimicrobial Resistance (AMR) & Advancing Public Health Preparedness.” Including discussions by two former FDA Commissioners (Scott Gottleib, Mark McClellan), the webinar will focus on the policy issues around AMR. Go here for the agenda and to register.
- 13 May 2021 (virtual, 9.30-11.00a EST): CDC-sponsored webinar entitled “AMR in a Changed World: Building Resilient Systems for Today and Tomorrow.” Moderated by CDC’s Michael Craig, an international panel will discuss “where we go from here to address AMR after the COVID-19 pandemic.” Go here to register.
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 24-29 May 2021 (online and in Geneva): ESPID 2021, the 39th Annual Meeting of the European Society for Paediatric Infectious Diseases. Save-the-date announcement is here, details to follow.
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 20-24 Jun 2021 (virtual, various times): World Microbe Forum sponsored by the American Society for Microbiology (ASM) and the Federation of European Microbiological Societies (FEMS). Go here for more details and to register.
- 27 Jun-2 Jul 2021 (Ventura, CA): Gordon Research Conference entitled “Antimicrobial Peptides”. Go here for details, go here for the linked 26-27 Jun Gordon Research Seminar that precedes it.
- 9-12 Jul 2021 (virtual): Annual ECCMID meeting (#31)
- 26 Jul-30 Jul 2021 (online): Small World Initiative Instructor Training Workshop – training for undergraduate professors in the wet lab techniques, parallel curricula, & pedagogical instruction to engage students in the hunt to find new antibiotic-producing soil microbes. Go here to register.
- 14-29 Aug 2021 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 11-15 Oct 2021 (physical, somewhere in the UK): UK-focused Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. Go here for more details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students. Bonus feature: For obvious reasons, the course didn’t happen in 2020! But as a celebration of the course’s 5th year, a webinar version was held on 29 Oct 2020: go here to stream it.
- 25-28 Oct 2021 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.