Dear All (this newsletter experiments with an Executive Summary/Details format; let me know what you think),
(Executive Summary) As a further support for implementation of recommendations from the UN Interagency Coordination Group on Antimicrobial Resistance (IACG AMR), the Tripartite Plus (WHO-FAO-OIE-UNEP) are seeking to create a Partnership Platform that will “engage institutions representing states, markets and civil society to shape a shared global vision and work on specific actions in common cause.” In effect, the Partnership Platform would be (should be) a global communication tool connecting diverse stakeholders. As part of the startup, the Tripartite Plus are running a survey to collect views on how best to create such a platform. Here are the links you need:
- A web page introducing a survey on the idea of the Partnership Platform
- Summaries of the 15-minute survey in English, French, and Spanish so that you can prep your answers
- The survey itself in English, French, or Spanish
Well done, Team Tripartite Plus! The survey closes on 18 Sep 2021: please sharpen your pencils and share your thoughts.
—
(Detailed version: a history lesson plus decoding of acronyms) Following on from the 2016 Political Declaration on AMR by the UN General Assembly (UNGA), the UN Interagency Coordination Group on Antimicrobial Resistance (IACG AMR) was convened in 2017. In April 2019, the IACG transmitted its pivotal report, “No Time to Wait: Securing the Future from Drug-Resistant Infections” to the UN Secretary-General. The report had recommendations in five key areas:
- Accelerating progress in countries,
- Innovating to secure the future,
- Collaborating for more effective action,
- Investing for a sustainable response, and
- Strengthening accountability and global governance.
Today’s newsletter is about element #5, accountability and global governance. On this front, IACG had four specific recommendations (and if you want more detail, see this excellent overview of the whole process as well as this May 2021 WHO newsletter):
- Strengthen joint One Health action based on target-setting, country priorities and needs
- Create a One Health Global Leadership Group on AMR supported by a joint secretariat managed by the Tripartite
- Key bit of political language: The Tripartite is WHO (human health) + FAO (food security) + OIE (animal health); UNEP (environment) recently jointed the Tripartite and thus current documents refer to the the “Tripartite Plus.”
- Convene an Independent Panel on Evidence for Action
- Expedite the member state-led work on a global development and stewardship framework to combat AMR
So, this brings us today. All of the steps are now underway (and I’ve above bold-faced those most relevant to this newsletter):
- Work is ongoing to support implementation of national action plans; see also this tracking database.
- The joint secretariat managed by the Tripartite Plus was created in 2019; see this excellent slide deck on the AMR Tripartite Plus.
- Created in 2020, the Global Leaders Group (GLG) is a high-level political leadership group chaired by two Prime Ministers (Bangladesh, Barbados).
- The GLG has the critical goal of maintaining urgency, political support, and momentum (see their 2021-2022 priorities) so that AMR remains front and center in the global political agenda (rather than being displaced by a subsequent crisis du jour).
- Of note, Industry are represented on the GLG by Merck’s Julie Gerberding, former director of the US CDC.
- Draft Terms of Reference for an Independent Panel on Evidence on AMR were released in 2020 and it appears that this panel is gradually coming together (see point 4 in this April 2021 UNGA summary).
- To my eye, the Independent Panel on Evidence for AMR appears similar in concept to the Intergovernmental Panel on Climate Change (IPCC) — let’s hope that it has a similar impact by generating objective, high-quality data to answer some of the unanswered questions about AMR!
—
Inhale, exhale … but wait, there’s more … and we’re about to get to that survey! As a further support for communication and collaboration, the Tripartite Plus are seeking to create a Partnership Platform that will “engage institutions representing states, markets and civil society to shape a shared global vision and work on specific actions in common cause.” To that end, the Tripartite Plus want to hear your views on how best to create such a platform. Here are the links you need:
- A web page introducing a survey on the idea of the Partnership Platform
- Summaries of the 15-minute survey in English, French, and Spanish so that you can prep your answers
- The survey itself in English, French, or Spanish
The ideas behind the Partnership Platform were initially unclear to me … how could this possibly work? Who is talking to whom? But I began to understand with Question 4 of the survey where we are told:
- Membership of the Platform is open to government representatives; UN agencies, international, intergovernmental and regional organizations; international and regional financial institutions, philanthropic donors; civil society organizations and networks relevant to AMR; academic and research organizations across the One Health spectrum relevant to AMR; private sector partners representing sectors that affect, or are affected by, AMR. Net: Everybody is included!
- The Platform’s membership shall include sectors or disciplines that are underrepresented in the other global governance mechanisms for AMR, e.g. civil society, private sector and financial institutions. Members of the Platform will focus on bringing in local and sectoral knowledge and power to support collective actions to tackle AMR. Net: Those who usually don’t have a voice are specifically sought!
This graphic in Question 13 then brought it all together for me:
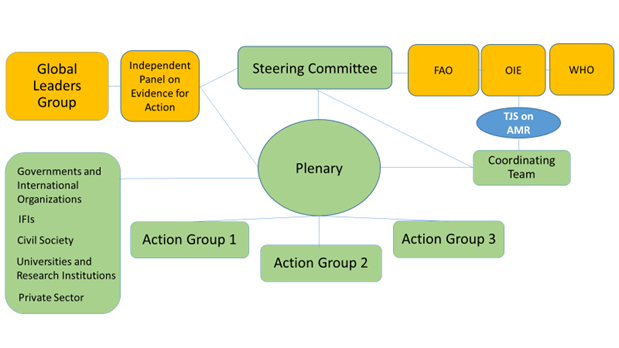
You’ll now spot all the actors in orange (the GLG, the independent panel, the Tripartite Plus; UNEP needs to be added to the figure, I think) and the joint secretariat (blue) surrounding the green bubbles of the elements of this partnership platform. We also can now understand the further text in Question 4:
- Members’ participation in the Platform for the purpose of achieving joint action will be channeled through:
- Action Groups developed to drive forward specific topics and subtopics involving different Cluster Groups;
- Cluster Groups that should ensure similar stakeholders are able to voice their views through the cluster and be represented in the Action Groups
—
Yow! The idea of a global conversation on AMR between so many diverse stakeholders is astounding! There is the risk that it becomes a giant talking shop with no action, but you miss 100% of shots not taken … this is certainly ambitious and worthy!
Well done, Team Tripartite Plus! The survey closes on 18 Sep 2021: please sharpen your pencils and share your thoughts. Everyone’s voice is needed!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- [NEW] The AMR Industry Alliance is offering a Stewardship Prize of 12.5k CHF (~$13.5k at current rates) to recognize innovative new approaches to AMR stewardship in Low- and Middle-Income Countries (LMICs). Go here for more details Applications are due by 8 Oct 2021.
- [NEW] The AMR Action Fund is now open to proposals for funding of companies with Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- [NEW] INCATE (Incubator for Antibacterial Therapies in Europe) is a newly launched early-stage funding vehicle. Details are still coming into focus, but per comments on 25 Aug 2021 at the BIOCOM conference, their goal is to support ~4 companies per year with about $250k/company. Contact details are on their website (https://www.incate.net/).
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 24-26 Aug 2021 (virtual, EU-centered timings): The 5th edition of the annual AMR conference sponsored by the BEAM Alliance, CARB-X, the Novo REPAIR Impact Fund, the IMI Accelerator, and the European Biotechnology Network. The in-person version of this meeting is consistently excellent; the video-based version will have to do for 2021. Go here for details.
- 30 Aug – 1 Sep 2021 (virtual, East Coast US timings): FDA (CBER)-NIAID-sponsored workshop entitled “Science and Regulation of Bacteriophage Therapy.” Go here for details and to register.
- 1-2 Sep 2021 (virtual, 10a-3p EST on both days): FDA -sponsored workshop entitled “Advancing the Development of Pediatric Therapeutics (ADEPT 7) Complex Innovative Trial Design.”
- In brief, “The Complex Innovative Trial Design Pilot Meeting Program (CID Program) facilitates and advances the use of complex and innovative trial designs that have the potential to optimize drug development in small populations.
- “Innovations that have been proposed include Bayesian and other methods of utilizing external historical information from previous pediatric trials or other populations (such as adults), adaptive designs, bridging biomarkers, etc.”
- “The workshop will specifically focus on two topics of interest: bridging biomarkers in pediatric extrapolation and Bayesian techniques in pediatric studies. In addition, the workshop will allow for an open dialogue around the use of these approaches among regulators, industry, academia, and patient organizations.”
- Looks fascinating. Go here for the detailed Federal Register notice and here to register.
- [NEW] 8 Sep 2021 (virtual, 9.30-11.00a CEST): GARDP/REVIVE-sponsored webinar entitled “Combination antibiotic therapy against drug-resistant Gram-negative bacteria: where the evidence stands.” Go here to register.
- [NEW] 9 Sep 2021 (virtual, 2-3p CEST): Webinar entitled “Antimicrobial Resistance: Turning Plans into Action” sponsored by the Uppsala Health Summit. Starting in 2014, UHS has convened a series of fascinating and diverse policy discussions (e.g., healthy urban chlidhoods). For their 2021 edition, they focused on Managing Antimicrobial Resistance through Behaviour Change. As I find the idea of behavioral approach to AMR to be fascinating and inadequately discussed, this webinar to report looks fascinating. Go here to register.
- 29 Sep-3 Oct 2021 (virtual, various times): 10th annual IDWeek conference. Go here to register.
- 30 Sep 2021 (virtual, 9a-noon EST, 2-5p BST): Featuring Dame Sally Davies and Marc Mendelson, this is a Vivli-sponsored workshop entitled “A Foundation Briefing on Industry AMR Surveillance: Data for Action” that will discuss Vivli’s new surveillance sharing platform. Go here to register.
- 8 Oct 2021 (Boston, in person, 9a-6.30p, COVID vaccination required): 8th annual BAARN (Boston Area Antimicrobial Research Network) meeting. Go here for details; registration link is pending.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 11-15 Oct 2021 (physical, somewhere in the UK): UK-focused Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. Go here for more details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students. Bonus feature: For obvious reasons, the course didn’t happen in 2020! But as a celebration of the course’s 5th year, a webinar version was held on 29 Oct 2020: go here to stream it.
- 22 Oct 2021 (virtual, 3:30 PM CET/9:30 AM ET): CARB-X webinar entitled “European Celebration of 5 Years of Progress with CARB-X: A virtual discussion of progress and plans to accelerate innovation globally.” Don’t be thrown by the word ‘European’ in the title — the early agenda does have a European flavor but the implications are global. Go here to register.
- 5-8 Nov 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.
- 9-13 May 2022 (Athens and online): 40th Annual Meeting of the European Society for Paediatric Infectious Diseases, Go here for details.
- 20-24 Sep 2022 (New Delhi): 21st Congress of the International Society for Human and Animal Mycology (ISHAM). Go here for details.
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.