Dear All,
Last fall, the team at ATMF (Access to Medicine Foundation) released the 3rd update to the AMR Benchmark series and Damiano de Felice was a guest author for a newsletter about the findings. That report offered a wealth of granular data, in-depth analyses and revealing graphs about what pharmaceutical companies in the global antibiotic and antifungal markets have been doing to tackle the rise of AMR and the global need for appropriate access to antimicrobials. The report can be summarized as “Clear signs of progress but still a lot to be done!” If you need a refresher on the 2021 report, you can quickly get up to speed by watching these videos I made with Fatema Rafiqi.
Now the ATMF team released a report on progress by companies to improve access to antibiotics and antifungals in lower middle-income countries (LMICs). The report also does a deep dive on strategies companies can employ to improve access and it provides 6 case studies that show how companies are making access happen. There are also some excellent graphics like the one below:
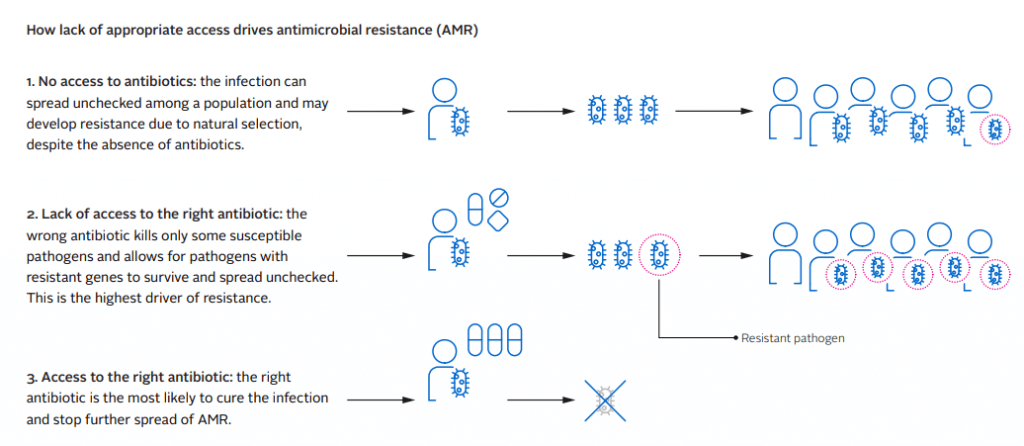
The report covers a range of options available to companies to increase access, recommendations to key stakeholders, and includes details on systemic factors that could be hindering progress on resolving this issue. You really ought to take the time to read this report yourself but, as always, here are my key highlights:
- ATMF has created a “toolkit of access strategies for companies” (with a helpful diagram!) to provide clear pathways for improving access.
- Since companies are drawn to large markets, they need to develop an understanding of localized smaller markets before introducing any generic antimicrobials. Take advantage of surveillance programs run by governments!
- Move away from single-source or limited supply chains when possible. Collaboration with other companies and manufacturers can help with this.
- Setting up local manufacturing is highly encouraged and instantly improves access.
- Use (non-exclusive) voluntarily licensing to allow local generic medicine manufacturers to produce and sell on-patent products.
But, other players have roles as well … companies can only do part of the work needed to ensure access. That theme was discussed in a 22 Mar 2021 newsletter and related video about the Stewardship and Access Plan Development Guide developed by CARB-X along with its funding partners. On this topic, the ATMF report helpfully discusses actions need from the rest of the ecosystem (this is a brief list — see the report for more details):
- Governments: Create treatment guidelines, stewardship plans, and surveillance systems! In high-income countries, implement Pull models such as the one being piloted by the UK
- Regulatory agencies: Harmonize regulatory schemes to ease registration across companies
- International health organizations: Partner with local governments and pharma to ensure access; build local manufacturing hubs; raise awareness of WHO’s prequalification system
- Healthcare professionals: Follow guidelines; work to define demand for new therapies
I applaud this broad perspective and especially the further call for delinked Pull incentives! To quote ATMF’s Marijn Verhoef, “There is no time to lose. After the inequity in global access to COVID-19 vaccines, the world’s poorest people must not be left at the back of the when it comes to fighting antibiotic resistance.“
Well done Team ATMF! Thank you for your hard work! –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- The AMR Industry Alliance has announced their 2022 Stewardship Prize! The program offers 10,000 CHF to recognize established, innovative approaches to AMR stewardship in low- and middle-income countries (LMICs). The 2021 prize went to the Infectious Diseases Institute (IDI) in Kampala, Uganda for their best practices in diagnostic stewardship and for their patient awareness campaigns dedicated to decreasing inappropriate use of antibiotics in their specialist HIV clinic in Kampala. Applications for the 2022 prize are due August 31, 2022. Thinking in terms of stewardship, WHO have recently released a pair of courses through the OpenWHO platform:
- Course 1 provides training on the WHO Policy Guidance on Integrated Antimicrobial Stewardship Activities
- Course 2 focuses on the WHO practical toolkit for antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries
- The online training courses can be found on the dedicated channel: https://openwho.org/channels/amr
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is a newly launched early-stage funding vehicle. Details are still coming into focus, but per comments on 25 Aug 2021 at the BIOCOM conference, their goal is to support ~4 companies per year with about $250k/company. Contact details are on their website (https://www.incate.net/).
- New funding rounds from CARB-X are expected soon now that funding for the next 10 years has been announced! [NEW] There is a 9 June 2022 webinar (9-9.30a ET) that will “discuss the global health issues of AMR, and how CARB-X and its partners are working together to address them.” Go here to register.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- [Not to be missed!] 8 Dec 2021: “The New Winds Pushing and Pulling Antibacterial Development.” This FABULOUS program featured talks from the UK team behind the NHS “Netflix” pilot, Kevin Outterson’s recently released report documenting the need for global Pull incentives to have a value of $2.2 – 4.8b, and speakers covering PASTEUR and work in the EU on pull incentives. The video is here — please make time to listen to this program!
- [Required reading!] The stunning 4 Feb 2022 webinar for the GRAM report (Global Research on Antimicrobial Resistance “1.27 million deaths per year are directly attributable to AMR”) is now available for replay. #AMRSOS!
- 8-9 Jun 2022 (virtual, 15.00-18.00 CEST / 9.00-12.00 EDT on both days): “Expert Workshop on Monoclonal Antibodies for AMR Pathogens” sponsored by the Paul-Ehrlich-Institut, on behalf of the COMBINE project. Day 1 focuses on preclinical development and translation (register here); Day 2 focuses on recurring problems and mitigation strategies in the clinical development (register here).
- 9 Jun 2022 (virtual, 9-9.30a ET). CARB-X-sponsored webinar at which CARB-X, BARDA, Wellcome and NIAID to discuss the global health issues of AMR, and how CARB-X and its partners are working together to address them.” Go here to register.
- 13 or 14 Jun 2022 (virtual, 14.00-17.00 (CEST) on 13 Jun, 09.00-12.00 (CEST) on 14 Jun, same agenda both days): “Supporting measures to mitigate AMR in One Health settings,” webinars sponsored by JPIAMR and ICARS that will consider at length the ‘Prevention and Intervention’ pillar of the JPIAMR’s Strategic Research and Innovation Agenda. Go here for details and to register.
- 16-18 June 2022 (Perth, Australia): Australasian Society for Infectious Diseases Annual Scientific Meeting is a hybrid event for adult and pediatric infectious disease and clinical microbiology specialists. Go here for details.
- 21 Jun 2022 (virtual, 10:00-11:00 ET | 15:00-16:00 BST): Launch of the AMR Register. Sponsored by Vivli with funding from many partners, this is the launch of an open-access repository for industry-generated surveillance data. Looks interesting! Go here to register.
- 22-23 Jun 2022 (virtual, 10a to approx. 2.30p ET on both days): Workshop entitled “Strategies for Early-Stage Programs Developing Novel Antibacterial and Antifungal Drugs.” Sponsored by NIAID’s Bacteriology and Mycology Branch (BMB), this 2-day webinar features a very strong faculty (including speakers from FDA) discussing tips and insights for early product discovery including in-depth discussions of funding opportunities. The timing is US-centered but video replay will be available. Do not miss this! Go here to register.
- 23 Jun 2022 (Virtual, 2-4p CEST): “Tackling the emerging threat of fungal drug resistance,” a webinar sponsored by JPIAMR, the Israeli MoH, and the UK MRC. Go here to register. Yes, that’s the Israeli Ministry of Health getting into AMR … perhaps in part stimulated by a 2015 report on the significant burden of fungal infections in Israel.
- 6 July 2022 (virtual, 15:00 CET/ 09:00 ET): WHO webinar entitled “Enhanced use of data to monitor safety and effectiveness of paediatric medicines”. Go here for details.
- 11-14 July 2022 (Sydney): Australian Society for Microbiology Annual National Meeting is a hybrid event that will feature a range of lectures and symposium sessions, as well as extensive opportunities for networking. Go here for details.
- 26 July 2022 (virtual, 10a-11.30a ET): REVIVE webinar entitled “New approaches for antibiotic discovery”. Go here for details.
- 24-27 July 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.
- 28-31 July 2022 (Singapore): 10th International Congress of Asia Pacific Society of Infection Control is a hybrid event for professionals in the Asia Pacific region. Go here for details and to register.
- 23 August 2022 (virtual, 11a-12.30p ET): REVIVE webinar entitled the Challenges and options in developing antibiotic combinations. Go here for details.
- 12-13 Sep 2022 (virtual, 9a-5p ET): This meeting of PACCARB is going to “identify key issues and critical policy gaps through a series of facilitated discussions examining a hypothetical large-scale disease outbreak scenario based on historic examples and estimates of future AMR outbreaks.” Sounds like pandemic wargaming (Center for Health Security; pre-COVID 19 May 2020 NPR article) to me! Go here for details.
- 20-24 Sep 2022 (New Delhi): 21st Congress of the International Society for Human and Animal Mycology (ISHAM). Go here for details.
- 4-7 Oct 2022 (Dublin, Ireland): The 2022 ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. This is an excellent meeting, especially for developers … and if you’ve missed it, the recordings from the 2021 meeting are online. Go here for details on the 2022 meeting.
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- 15-23 Oct 2022 (in person, residential, Les Pensières, Veyrier-du-Lac, France): The 6th edition of Patrice Courvalin’s fabulous ICARe residential training course covering all things AMR is on for 2022! This is a soup-to-nuts training in AMR: it is very intense, very detailed, and always gets rave reviews from attendees. Registration is open 21 Mar 2022 to 21 June 2022 and is limited, so book your slot as soon as you can. Go here for details.
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 17-20 Nov 2022 (Kuala Lumpur, Malaysia): The International Congress on Infectious Diseases will take place for the first time as a hybrid event. Go here for details.
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.