Dear All, Two important reports for your awareness this afternoon.
First, the Global AMR R&D hub have responded to a request from the G7 Finance and Health Ministers to provide an update to their 2022 progress report in advance of upcoming G7 meetings. As background, recall that the Finance Ministers in 2021 (during the UK G7 Presidency) call for the G7 to take steps to address antibiotic market failure — and these progress reports are part of that process. Here are the links you need to the 2023 update as well as some relevant history:
- The new 2023 report’s website, the full report, and a 2-page summary
- The 2021 G7 Finance Minister’s call for Pull, the Hub’s 2022 report, and a 2022 G7 Health Minister call for Pull
It is very exciting to see that actions on AMR are underway in all of the G7 (note in particular the excellent country-by-country brief summary in the 2-page report as well as a superb table at the very end of the long report). But, there is always more to be done and the new report recommends 3 high-level priority actions: (1) Renew Priorities and Timelines, (2) Encourage Alignment and Targeted Action on Financing Mechanisms, including Push and Pull Incentives, and (3) Prioritise Equity and Global Access to Priority Antibacterials. Point (2) is obviously relevant to Pull! Good work, Team Hub!
—
Second, we have a reprise of the 2020 report on AMR from the US GAO (Government Accounting Office). The timing here is related to the reintroduction of the PASTEUR Act and as part of the 28 April 2023 hearing on AMR in the US House of Representatives (detailed newsletter, 30-minute YouTube discussion of recent events). GAO’s original report was released in Mar 2020 and sadly was drowned out by global discussions COVID-19. The GAO provided testimony as part of the 28 April hearing and thus had a chance to reprise their 2020 report.
The GAO report is an excellent survey of the overall problem with clear discussions of issues around surveillance, diagnosis, new therapies, and use. The report had 8 recommendations across these areas, with one recommendation focused on Pull: “… develop a strategy to further incentivize the development of new treatments for antibiotic-resistant infections, including through the use of postmarket financial incentives.” The questions “Why AMR?” and “How to Pull?” are nicely summarized in two graphics from the report.
Staring with “Why AMR?”, we have this very clear visual story:
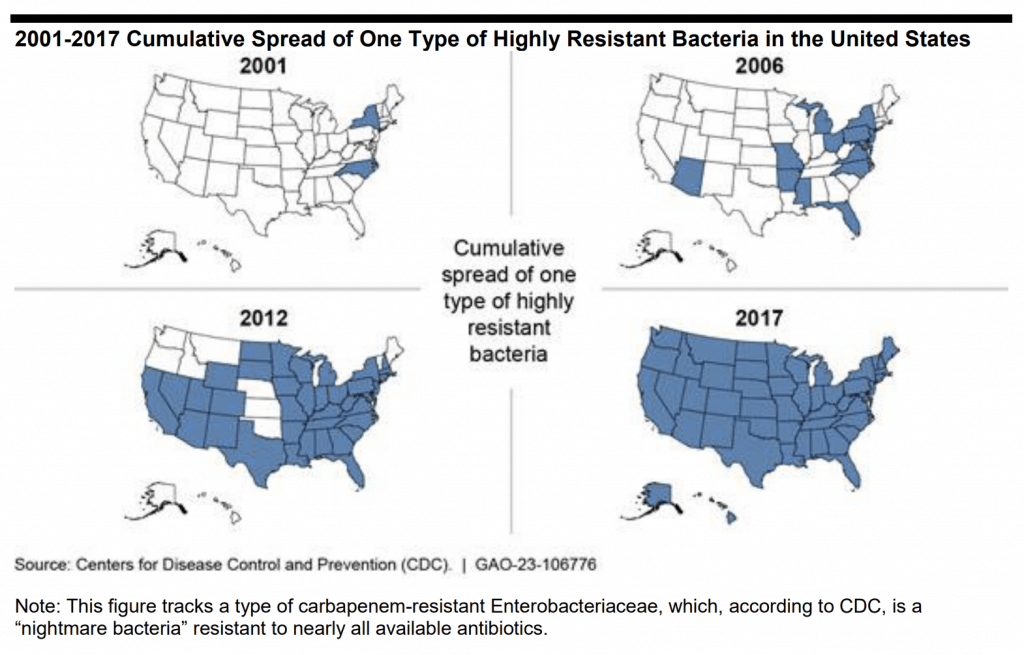
A picture is worth 1,000 words, no?
And as to “How?”…
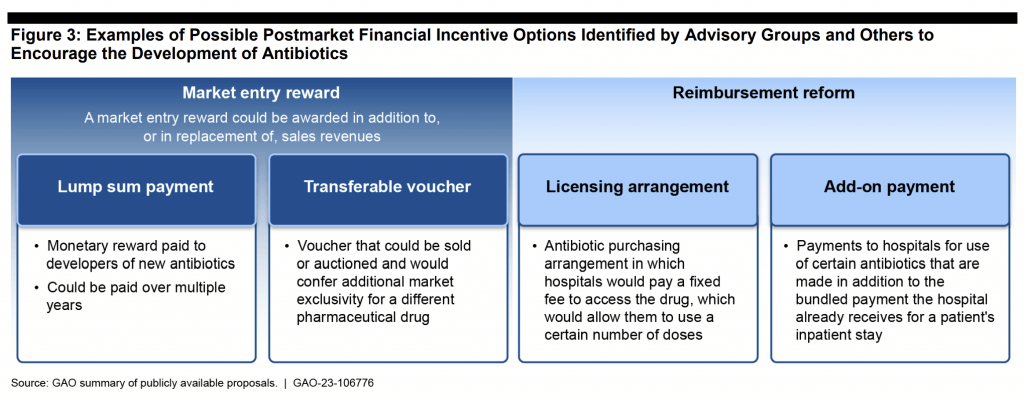
Here you will immediately recognize the familiar ideas of market entry rewards (e.g., transferrable exclusivity vouchers (now being pursued by the European Commission) and reimbursement reform (e.g., the UK “Netflix” purchasing model, the PASTEUR Act, and also part of the EC’s proposed program).
Great stuff! Many thanks to Team GAO for this excellent report!
—
To close, I’ll point you at the excellent 1-h Duke-Margolis 9 May 2023 webinar entitled “Drug-Resistant Infections & the Immediate and Future Demand for Novel Antimicrobials.” Chaired by Mark McClellan (Director of the Duke-Margolis Center), the webinar featured a 10-minute discussion with Sen. Todd Young (one of the sponsors of the PASTEUR Act) followed by a panel discussion between leading global thought leaders from academic medicine (Helen Boucher, also a former voting member of PACCARB), CDC (Michael Craig, Senior Advisor for Antibiotic Resistance), the European Commission (Annukka Ojala, Deputy Head of Cabinet), and the AMR Action Fund (CEO Henry Skinner). Please make time for this one … and fingers/toes crossed for the PASTEUR Act (US) and the proposed Pull incentives in Europe!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 3-5 Jul 2023 (Tours, France): 9th Symposium on Antimicrobial Resistance in Animals and the Environment (ARAE). Sponsored by INRAE (French National Research Institute for Agriculture, Food, and Environment, itself a merger of merger of INRA, the French National Institute for Agricultural Research, and IRSTEA, the French National Research Institute of Science and Technology for the Environment and Agriculture), this conference has been running since 2005. Go here for details.
- 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- [NEW CALL FOR PIPELINE SUBMISSIONS] 11-15 Oct 2023 (Boston, USA): IDWeek 2023, the annual meeting of the Infectious Diseases Society of America. Go here for details and to register. Also note that there is a call for IDWeek Pipeline submissions with a deadline of 17 May 2023: “Submit Your Novel Antimicrobial Agents or Diagnostic Technologies: Companies are invited to submit antimicrobials that are in preclinical stages of development (Phase II and III preferred) or were approved after January 2023 for a pipeline session at IDWeek that will include antibacterials, antifungals and antivirals (excluding COVID-19 and HIV). Companies developing novel diagnostic technologies with a minimum of some preliminary proof of concept data may submit as well.”
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.