Dear All,
Some great news today! We have visible evidence of global government action in support of the addressing our antibiotic gaps.
First, CARB-X has announced that Canada has now joined its coalition of funders with a pledge of $6.3m! Woot, woot! Go Team Canada!
Second, the UK and Germany have renewed their funding support with commitments of GBP 24m and EUR 41m, respectively. Building on support from the US Government (BARDA), Wellcome Trust, and the Bill & Melinda Gates Foundation, CARB-X looks well-placed to continue its efforts to build an innovative and diverse early pipeline of therapies and diagnostics.
And last (but not least!), I have become aware of updated details on the work by Japan’s MHLW (Ministry of Health, Labour, and Welfare) to advance its thinking on Pull mechanisms that would work in Japan. I missed it initially, but a post by CARB-X’s Damiano de Felice on TAFKAT provides a link to materials from a 29 March 2023 meeting of the MHLW’s Study Group on Market Incentives for Antimicrobials.
As Damiano says in his post, “The documents include preliminary ideas and suggestions about selection criteria, structure of the procurement contract, contractual obligations and evaluation process.” I do not speak Japanese, but with the help of Google Translate, I can provide further insights with this pair of translated images from slide 10 of this key document:

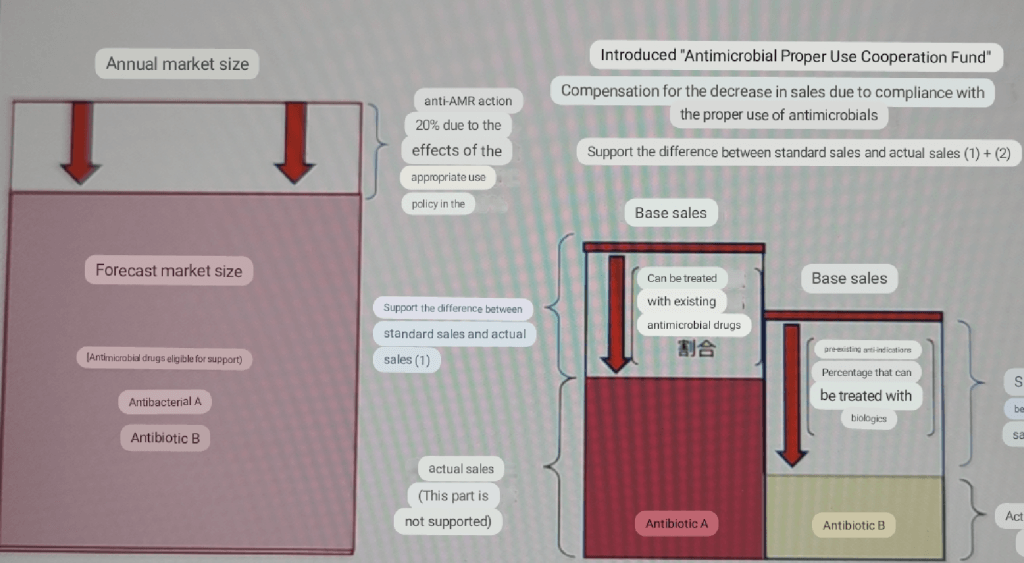
The text that begins “How about setting the scale of market incentives…” is positioned above the graphics showing the well-known economic paradox of new antibiotics. Reading the translation, the idea is that an “Antimicrobial Proper Use Cooperation Fee” spans the gap between sales that are (appropriately!) limited by stewardship and the societal value of the antibiotic. That’s very interesting language — “Proper Use Cooperation Fee” neatly encapsulates the concept of providing reimbursement to ensure availability.
22 Aug 2023 update: Helpful additional details on MHLW’s work-in-progress were provided in a 7 Aug 2023 newspaper article — see this newsletter for details.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- The AMR Industry Alliance has announced that applications are again open for its annual Stewardship Prize. The Alliance began the Stewardship Prize in 2021 to identify and support innovative approaches to combatting antimicrobial resistance in low-to-moderate-income countries — the winning application receives CHF 10,000. Applications close September 1, 2023; go here to see past winners and here to apply.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- [NEW and especially recommended for anyone who would like a good introduction to the AMR problem] 24 Aug 2023 (online, 6.45am EST, 12.45p CET, 4.15p India): Webinar entitled “Small and Medium Scale Antibiotic Developers, Challenges they face and the way forward.” Sponsored by the India-based CSE (Centre for Science and Environment, an NG based in New Delhi), this is the 2nd in a 3-part webinar series — go here to register. The first webinar on “The Crisis in Antibiotic R&D” can be seen on YouTube and was an excellent 90-minute tour of the general AMR problem on 28 July 2023. The webinar was held in conjunction with release of a report entitled “A Developing Crisis” by CSE, was hosted by Sunita Narain (Director General of CSE and a member of the Global Leaders Group on AMR), and featured a global roster of speakers from India, Europe, and United States. A 3rd webinar on solutions is planned.
- [NEW] 29 Aug 2023 (online, 1-2p EST): Webinar entitled “Preparedness to Combat Infectious Disease and Drug-Resistant Bacterial Threats.” Sponsored by the Duke-Margolis Center for Health Policy, the webinar will focus on preparedness in the context of both viral and bacterial threats, including drug-resistant bacteria and antimicrobial resistance (AMR). Duke-Margolis webinars are hosted by Mark McClellan (founding Director of the D-M Center and formerly Commissioner of the US FDA) and are always good value. Go here for the full agenda and to register.
- 19-22 Sep 2023 (Boston, USA): ASM-ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. This is an excellent development focused meeting … highly recommended! Go here for details and to register.
- 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- 11-15 Oct 2023 (Boston, USA): IDWeek 2023, the annual meeting of the Infectious Diseases Society of America. Go here for details and to register.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.
- 6-7 Feb 2024 (online): Antimicrobial Chemotherapy Conference. This is an annual, free of charge conference that is co-organized by GARDP and the British Society for Antimicrobial Chemotherapy (BSAC). Details to follow — for now, just mark your calendar.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- [NEW] 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.