Update on 11 October 2023: The annual BARDA Industry Day is 13-14 November 2023! You can attend virtually or in person at the Grand Hyatt Washington, DC. Click here for more details!
Dear All,
Hot on the heels of the BAA from BARDA and from ARPA-H, we now have a Broad Agency Announcement from the FDA! There will be a virtual launch day event on 25 October 2023. The event runs from 1p until 4:30p ET and is open to everyone with a special emphasis on small businesses!
I’ve not been able to dig deeply so here are the highlights:
- The FDA anticipates that research and development activities awarded under this BAA will serve to advance scientific knowledge to accomplish its mission to protect and promote the health of the USA.
- For the most up-to-date documentation, check out this FY24 FDA Broad Agency Announcement (BAA) for Advanced Research and Development of Regulatory Science page on SAM.gov.
- You can see the current agenda on this website and it looks like it will be a very informative session for anyone who is interested in applying.
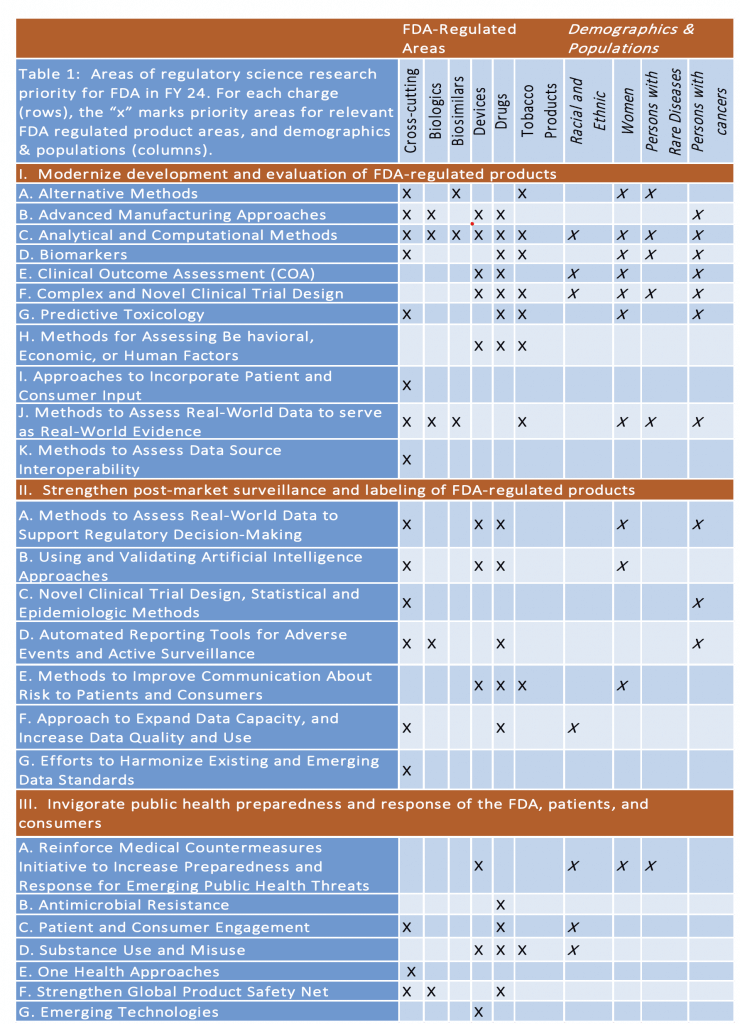
- Areas of interest are quite broad. Based on my scan, work on Biomarkers, Complex/Novel Trial Designs, and Real-World Evidence are all ideas that would be in scope and especially relevant to the AMR community — see the grid below my signature for more ideas!
- Optional Early BAA Concept Paper due date: 6 November 2023
- BAA Stage I Package due date: 19 February 2024
- 20 Oct 2023 Addendum: Please also note in particular that FDA’s Office of Infectious Diseases has an RFP focused on generating data to support urine-specific breakpoints for uncomplicated UTI (uUTI). Please see this newsletter.
Many thanks to our colleagues in the US Government for creating these opportunities! If you are interested, click here to register now and then share this email with someone else you think might be interested! The more who know about this development from the FDA, the better. There is no deadline for registering and, if you can’t make it for any reason, there will be a recording available afterwards.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Below: Grid from the BAA showing areas of interest:
Current funding opportunities (most current list is here)
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- ARPA-H has an Open BAA that is accepting applications through 14 March 2024. It is quite wide-ranging in its scope and definitely includes AMR-related projects. See this newsletter for discussion of the BAA and an AMR project that it now supports.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. Applications are accepted on a rolling basis; go here for all the details.
- The ENABLE-2 consortium has announced a call to support hit-to-lead compound development by researchers at publicly-funded European universities. The call is focused on molecules with the potential to be direct-acting therapies for one or more of the following priority pathogens: ESBL-producing/carbapenem-resistant Enterobacteriaceae (E. coli, K. pneumoniae), P. aeruginosa, A. baumannii, methicillin-resistant S. aureus, or vancomycin-resistant E. faecium. The Call is open continuously, applications are reviewed at intervals, and funding is non-dilutive. Expressions of interest received before 30 Sep 2023 would be considered in November 2023. Applications received after this date will be evaluated in the spring of 2024 (date to be decided). Go to https://www.ilk.uu.se/enable2/apply/ for further details.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- General note: Virtual meetings are easy to attend, but regular attendance at in-person events is the key to networking and deeper insight. My personal favorites for such in-person meetings are marked below as HIGHLY RECOMMENDED and are the BEAM Alliance’s AMR Conference (March, Europe), ECCMID (April, Europe), the ASM-ESCMID Developer’s meeting (September, alternates sides of the Atlantic), and ID Week (October, USA). Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 11-15 Oct 2023 (Boston, USA): IDWeek 2023, the annual meeting of the Infectious Diseases Society of America. Go here for details and to register. HIGHLY RECOMMENDED.
- 12 Oct 2023 (virtual, 2-3p CET) GARDP-sponsored webinar entitled “Market interventions to improve access to antibiotics for resistant infections.” Go here to register.
- [NEW] 18 Oct 2023 (virtual, 7.30a-9a EST): Webinar from CSE India entitled “Recognizing antibiotics as a global public good: challenges and possibilities”. Go here for details.
- [NEW] 19 Oct 2023 (virtual, 3p-4.30p CET): Webinar enititled “People-centred approach to addressing antimicrobial resistance in human health” as part of the WHO Global Webinar Series to Support Implementation of National Action Plans on Antimicrobial Resistance. Go here to register.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.
- [NEW] 7-9 Nov 2023 (Atlanta, USA): 13th Annual Antibiotics Symposium hosted by the National Institute for Animal Agriculture. Go here for details.
- [NEW] 14-15 Nov 2023 (Rockville, Maryland, USA and virtual): NIAID workshop entitled “Systematic Approaches for ESKAPE Bacteria Antigen Discovery”. Go here for details.
- 6-7 Feb 2024 (online): Antimicrobial Chemotherapy Conference. This is an annual, free of charge conference that is co-organized by GARDP and the British Society for Antimicrobial Chemotherapy (BSAC). Details to follow — for now, just mark your calendar.
- 6-7 Mar 2024 (Basel,[NEW] 6-7 Mar 2024): Sponsored by the BEAM Alliance, the AMR Conference is now in its 8th year and is consistently an excellent meeting for developers. You can’t register yet but you can mark your calendar and signup for notifications about the meeting. HIGHLY RECOMMENDED.
- 17-22 Mar 2024 (Ventura Beach, CA, in person): Gordon Research Conference (GRC) entitled “New Antibacterial Discovery and Development” with a 16-17 Mar 2024 pre-conference Gordon Research Seminar (GRS) for young doctoral and post-doctoral researchers. An intensive residential meeting, GRCs are highly recommended for networking and deep research insights. Apply here for the GRC and here for the GRS.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details. HIGHLY RECOMMENDED.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here for the meeting’s general website. You can’t register (yet) for the 2024 event, but you can mark your calendar. HIGHLY RECOMMENDED.