Dear All (unapologetically wonkish … very important material!),
Let’s set the scene today by considering two quotes:
- Prosaic: “The successful treatment of patients with cancer has long depended on the capacity to manage infectious complications.” (Shropshire 2025, cited below)
- Blunt translation: “Your cancer will be controlled, but then you may die of infection.” (Abdul Ghafur, quoted from a 2019 podcast entitled “Superbugs Force a Deadly Choice for Cancer Patients”)
In short, it is entirely possibly at present to be able to cure a cancer but be unable to cure the infection incurred along the way! Ugh!
Although the relevance of AMR to cancer is certainly not a surprise, the available data on the burden of AMR in cancer are generally small-scale reviews in HIC (High-Income Country) settings. As a step towards increased understanding that should both provoke further research as well as add patient voices to the call to action, we have today 3 recent papers and an excellent editorial commentary. Here are the links you need:
- AMR in hospitalized US patients with cancer: Gupta V et al. Burden of Antimicrobial Resistance in Adult Hospitalized Patients With Cancer: A Multicenter Analysis. Cancer Med. 2024;13(24):e70495 (https://doi.org/10.1002/cam4.70495).
- AMR in US outpatients with cancer: Gupta V et al. Incidence and prevalence of antimicrobial resistance in outpatients with cancer: a multicentre, retrospective, cohort study. The Lancet Oncology. 2025;26(5):620–8 (https://doi.org/10.1016/S1470-2045(25)00128-7).
- An enormous literature review: Sallah YH et al. Antimicrobial resistance in patients with haematological malignancies: a scoping review. The Lancet Oncology. 2025;26(5):e242–e52 (https://doi.org/10.1016/S1470-2045(25)00079-8).
- Last but not least, the editorial that ties the papers together: Shropshire WC and Shelburne SA. Antimicrobial resistance: a problem across the cancer care continuum. The Lancet Oncology. 2025;26(5):537–8 (https://doi.org/10.1016/S1470-2045(25)00155-X).
- 23 Sep 2025 addendum: Following a 25 Jun 2025 webinar, there is a 5-page summary of this research (webpage, 5-page .pdf).
I suggest you start your reading journey with the editorial — it provides a good tour. Then, the titles of the 3 primary papers are a good guide to what you find in each. As a survey to get you going, here are my notes:
The pair of papers by Gupta and colleagues (#1, #2) are US-based analyses of rates of resistant pathogens in both inpatients (#1) and outpatients (#2) with cancer:
- Commissioned by the Cancer & AMR Consortium, a collaboration between BD, UICC (Union for International Cancer Control), and the AMR Action Fund, the authors of this clever pair of papers have mined a large electronic dataset (the BD Insights Research Database) for the period 1 April 2018 to 31 Dec 2022 (almost 5 years).
- Per various descriptions in the papers, the BD Insights Research Database is a clinical research database covering both small and large hospitals as well as urban and rural areas within the US. Created by Becton, Dickinson and Company, Franklin Lakes, NJ, USA. it includes pharmacy, laboratory, and administrative data, admission, transfer, and discharge data feeds, and information on patient demographics.
- Using a standard rule for approximating unique isolates (reducing the data to the first source-specific pathogen within a 30-day window), the authors have inferred cancer vs. non-cancer status by considering case site and by examining pharmacy records for use of cancer vs. non-cancer drugs.
- Although this approach does have limitations (e.g., rates of sampling might be different in the groups, an isolate does not equal an infection, patient outcomes cannot be assessed), the approach allows consideration of a large amount of data while avoiding privacy concerns:
- Paper #1 considers data from 4.6m admissions, including 300m in patients with cancer!
- Paper #2 considered 1.7m total isolates of which 53k were from 27k cancer patients vs. 1.6m from 0.93m non-cancer patients!
- The data allow the authors to conclude that the rates of non-susceptible (NS) pathogens per 1,000 hospital admissions (#1) or 1,000 pathogens (#2) can be as much as 2- to 3-fold higher in cancer than non-cancer settings, especially for Enterobacterales (e.g., fluoroquinolone-NS, carbapenem-NS, and ESBL-producers), vancomycin-resistant Enterococcus, and MRSA. Interestingly, the rates of NS for Acinetobacter were similar to (or slightly lower than) in cancer patients.
- The authors conclude with a call for increased attention to the need for enhanced surveillance, infection prevention, and timely diagnostic stewardship to improve antibiotic prescribing in this population.
The 3rd primary paper is a massive literature review commissioned by the Union for International Cancer Control (UICC, https://www.uicc.org/). As the authors state in their introduction:
- “In 2020, the Union for International Cancer Control (UICC) established the Task Force on Antimicrobial Resistance and Cancer Care, comprised of experts in cancer care and infectious diseases, to provide guidance on best practices, identify research gaps, and mobilise the cancer community towards effective policy change.
- “However, literature gaps were identified on
- the global prevalence of AMR in patients with cancer,
- the cause of infections, and
- the consequences of AMR on cancer outcomes.”
As a start on rectifying these data gaps (and see also UICC’s great webpages on AMR), UICC commissioned a review to “assess the global prevalence of AMR and its consequences on patients with haematological malignancies.” The strength of this study is that the global approach to the literature review allows consideration of AMR rates in all parts of the world, at least to the extent that data have been published. Starting with just over 16k studies during 2000-2023 (that’s 24 years of data!), just over 16k studies were winnowed down to 274 that provided sufficient data for analysis. From these papers, the authors conclude (quoting from their abstract, but also abbreviating a bit):
- “The prevalence of AMR bacterial infections from seven WHO priority pathogens in patients with haematological malignancies was 35% (95% Confidence interval = 30–40%).
- “The most frequent AMR infections reported were bloodstream infections, with the highest reported AMR pathogens in third-generation cephalosporin-resistant Enterobacterales (pooled prevalence rate 44% [23–64%]), MRSA (43% [31–54%]), and vancomycin-resistant enterococci (41% [26–56%]).
- “53 (65%) of the 81 studies that reported mortality showed higher mortality rates associated with AMR infections.
- “168 (61%) studies were conducted in high-income countries, with no studies published from the WHO Africa region, revealing a substantial data gap from low-income and middle-income regions.”
As a way to sum up the entire story, let’s consider two figures from Sallah et al. The first shows the geographic sources of their data:
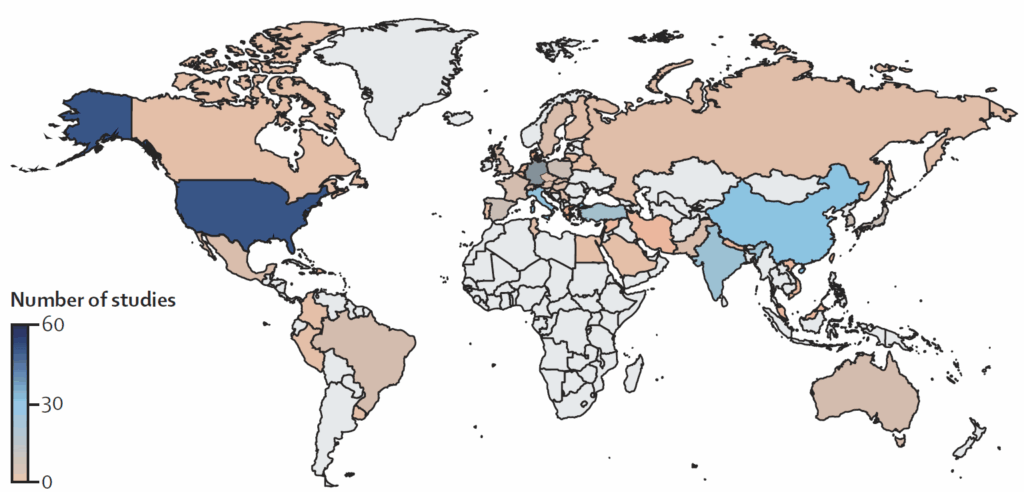
And I think that’s pretty self-explanatory! Data in HIC? Yes, often. Data in LMICs? Not so much!
And then we have this figure. Note that it is NOT a comparison of cancer vs. non-cancer — rather, it is simply the aggregate rate of resistant pathogens from 24 years of data on cancer patients. Look closely at the legend at the top — the stated n is the number of papers covering each pathogen):
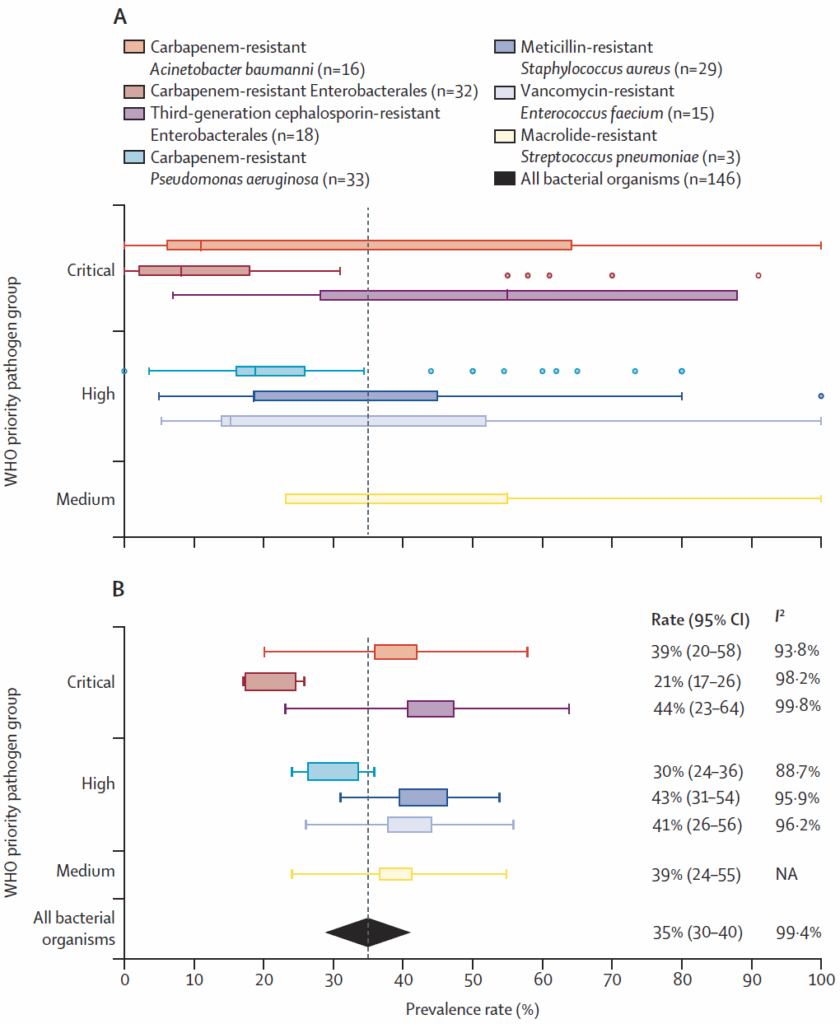
The data are shown first (A) as a box-and-whisker plot and then (B) as a forest plot of pooled prevalence of antimicrobial-resistant organisms.
—
Whew! I know that’s a lot to consider and there are many conclusions that could be drawn. But, let’s go with a simple one that is evident from the figure above: rates of resistance in key pathogens (e.g., ESBLs, fluoroquinolone-NS Enterobacterales) consistently exceed the 10% threshold commonly used as a point at which empirical therapy should be adjusted. And as the newer agents needed for these pathogens are not universally available, choices will be reduced to older, more toxic agents that are linked to higher mortality (see our recent discussion of DTR, Difficult-to-Treat Resistance, 25 April 2025 newsletter).
In summary, the authors of these papers are to be thanked for providing strong data. The idea that your cancer might be controlled but you could suffer with (or die from) an infection is simply not acceptable!
The authors have also shown us many areas for fruitful research: (i) the papers by Gupta et al. point out the possibilities in mining a large dataset and (ii) Yallah et al. highlight the (many!) research gaps.
Finally, these data should be a call to the cancer community to join the AMR community in pressing for global action — the voices of many are needed to ensure a sustained global push to ensure we have adequate antibiotics for now and for future generations. The intersection of AMR and cancer should be especially relevant given this year’s UN High-Level Meeting on noncommunicable diseases (NCDs), following last year’s High-Level Meeting on AMR.
With thanks to these authors and all best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- HERA posted a call on 24 Mar 2025 entitled “Development of a Rapid Point-of-care Antimicrobial Susceptibility Testing Diagnostic Medical Device (HADEA/2025/CPN/0006).” In it, they call for tenders under which they will provide up to 13m EUR for development of a point-of-care diagnostic medical device that can provide antimicrobial susceptibility results on the bacteria or fungi causing an infection in humans, within one hour or less from subject sample collection, and ideally, to also allow for pathogen identification. To apply, you must submit a request to participate by 12 May 2025; selected candidates who met the eligibility criteria will be able to submit a full technical tender. Go to the EU Funding and Tenders Portal to apply; see also the 19 Feb 2025 newsletter for details.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X will have two calls during 2025 that span two areas: (i) Small molecules for Gram-negatives (the focus is on Pseudomonas aeruginosa) and (ii) Diagnostics for typhoid (the focus is diagnosis of acute infections in 60 minutes or less). See this 26 Feb 2025 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. The first cycle will accept expressions of interest during the window 16-30 April 2025; the 2nd round will be open 1-12 Dec 2025.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are BEAM’s AMR Conference and GAMRIC (formerly, the ESCMID-ASM conference series). Hope to see you there!
- [UPDATED LOCATION – To maximize options for global attendees, the conference location is now central London, UK] 1-3 Oct 2025 GAMRIC, the Global AMR Innovators Conference (London, UK). Formerly the ESCMID-ASM Joint Conference on Drug Development for AMR, this meeting series is being continued under the joint sponsorship of CARB-X, ESCMID, BEAM Alliance, GARDP, LifeArc, Boston University, and AMR.Solutions. The ongoing series will continue the successful format of prior meetings with a single-track meeting and substantial networking time (go here to see details of the outstanding 2024 meeting). Registration will open on 5 May 2025; in the interim, the preliminary agenda can be found at that same link (https://www.gamric.org/). The meeting will be limited to approximately 300 attendees, so please be sure to register promptly to avoid disappointment! The abstract submission window will run 5 May to 13 June and an application round for travel grants is expected to run in a similar time frame.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society of America. Details pending; go here for the general meeting website.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference. Sponsored by the BEAM Alliance, the 9th AMR Conference has just concluded and it’s again been an excellent meeting! Please mark your calendar for next year. You can’t register yet, but details will appear here!
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. You can’t register yet, but you can go here for details on the outstanding 2025 meeting.
Upcoming meetings of interest to the AMR community:
- [NEW] 22 May 2025 (9.30-11.00a CEST, virtual): GARDP REVIVE webinar entitled “Post-licensing clinical trials for advancing the use of antimicrobials.” Go here for details and to register.
- 19-23 June 2025 (Los Angeles): ASM Microbe, the annual meeting of the American Society for Microbiology. Go here for details.
- 10-13 Sep 2026 (Lisbon, Portugal): 6th ESCMID Conference on Vaccines. Go here for details.
- [UPDATED LOCATION] 1-3 Oct 2025 GAMRIC, the Global AMR Innovators Conference (London, UK; formerly the ESCMID-ASM Joint Conference on Drug Development for AMR). See list of Top Recurring meetings, above..
- [Application season is open — I am late in noticing!] 11-19 Oct 2025 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2025 will be the 9th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications are being accepted from 20 Mar to 21 Jun 2025 — go here for more details.
- [NEW] 17-20 Sep 2025 (Porto, PT): 14th International Meeting on Microbial Epidemiological Markers (IMMEM XIV). Go here for details.
- [NEW] 9-13 Nov 2025 (Portland, OR, USA): ASM Conference on Biofilms. Go here for details and to register.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025. See list of Top Recurring meetings, above.
- [NEW] 29-31 Oct 2025 (Bengalaru, India): ASM Global Research Symposium on the One Health Approach to Antimicrobial Resistance (AMR), hosted in partnership with the Centre for Infectious Disease Research (CIDR) at the Indian Institute of Science (IISc). Go here for details and to register.
- [NEW] 28-30 Jan 2026 (Las Vegas, NV, USA): IDSA and ASM have just announced (7 May 2025) a new US-based meeting series entitled IAMRI (Interdisciplinary Meeting on Antimicrobial Resistance and Innovation) and described as a “forum for collaboration and exploration around the latest advances in antimicrobial drug discovery and development.” You can’t register yet but you can go here to see general details about the new meeting.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference sponsored by the BEAM Alliance. See list of Top Recurring meetings, above.
- 8-13 Mar 2026 (Renaissance Tuscany Il Ciocco, Italy): 2026 Gordon Research Conference (GRC) entitled “Antibacterials of Tomorrow to Combat the Global Threat of Antimicrobial Resistance.” A Gordon Research Seminar (GRS) will be held the weekend before (7-8 Mar) for young doctoral and post-doctoral researchers. Space for the GRS and the GRC is limited; for details and to apply, go here for the GRC and here for the GRS.
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
Self-paced courses, online training materials, and other reference materials:
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.
- GARDP’s REVIVE website provides an encyclopedia covering a range of R&D terms, recordings of prior GARDP webinars, a variety of viewpoint articles, and more! Check it out!
- GARDP’s https://antibioticdb.com/ is an open-access database of antibacterial agents.
- The CARB-X website provides a range of recordings from its webinars, bootcamps, and more. A bit of browsing would be time well spent!
- British Society for Antimicrobial Chemotherapy offers an eLearning section: Education – The British Society for Antimicrobial Chemotherapy.