Dear All:
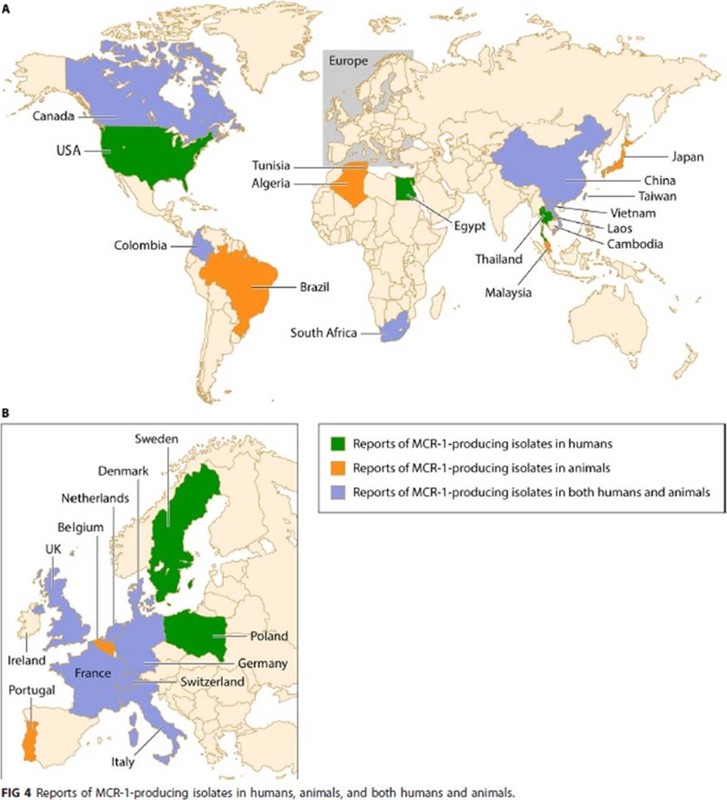
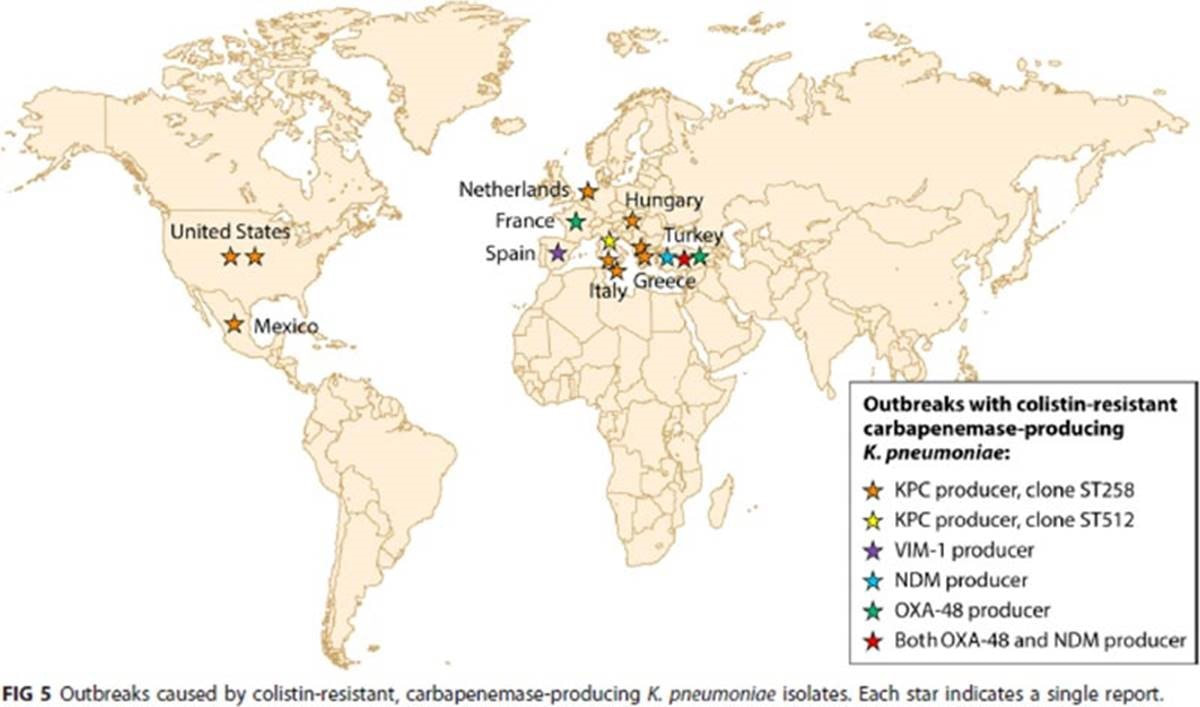
There’s a very comprehensive review of the polymyxins by Poirel, Jayol, and Nordmann just out in Clin Micro Reviews (https://journals.asm.org/doi/10.1128/CMR.00064-16). If you’re a fan of good quality graphics as way to tell the story of AMR, their Figure 4 is worth capturing! Ditto their Figure 5 of outbreaks of colistin-resistant, carbapenemase-producing K. pneumoniae. Both are shown below.
This paper also reminds me of Marson 2016 (JAMA 316:1193-204, 2016) in which the NIAID team provides two further useful graphics showing how rapidly AMR can develop and spread. Good graphics like these are so very helpful in communication!
Would all of you who are working on new products for Gram-negative rods please get really, really, REALLY busy?
Best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Chief Strategy Officer, CARB-X | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx
Figures 4 and 5 from Poirel L, Jayol A, Nordmann P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin Microbiol Rev. 2017;30(2):557-96.
Below left: Time (years) to resistance for new classes. Below right: Recovery of mcr-1-expressing E’bacteriaceae. From Marston HD et al. Antimicrobial Resistance. JAMA. 2016;316(11):1193-204.