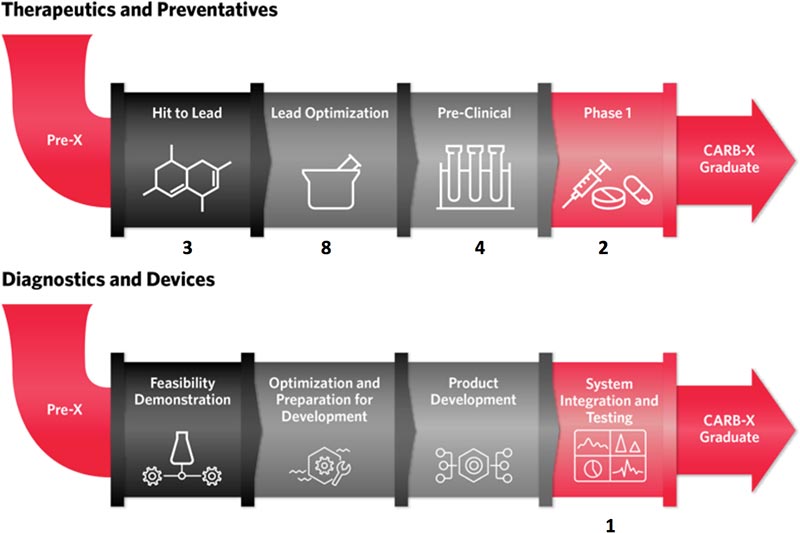
Dear All: CARB-X has today announced funding of 7 more companies, bringing the total funded portfolio to 17 potential new therapies (or preventatives) and 1 novel point-of-care diagnostic (see graphic above). Befitting the international nature of those of you who read my occasional notes (see map just below), there are now funded companies in 6 countries (US, UK, France, Switzerland, Ireland, and India).
Above: Locations of recent readers of these newsletters. Darker = more readers
You can read more in the press release (and don’t miss the two interactive & clickable portfolio tables), but I think it is particularly exciting that the 7 new projects include five potential new class antibiotics for Gram-negative bacteria. These will take a long time to mature and only a few will ultimately succeed, but the therapeutic part of the portfolio now contains 10 projects that pursue a novel target and 8 that are potential novel class small molecules.
All best wishes, –jr
PS: For those of you who miss the broadcast or don’t have a local PBS station, the FRONTLINE episode of Hunting the Nightmare Bacteria will be available for streaming at http://www.pbs.org/wgbh/frontline/ beginning sometime in the next 24h.
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Chief Strategy Officer, CARB-X | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://13.43.35.2/blog.html
Upcoming meetings of interest to the AMR community:
- 5-8 Sep 2017: ASM-ESCMID conference on antibiotic R&D, including the CARB-X + GARDP Antibiotic Bootcamp
- 13 Sep 2017 (DC): FDA-CDRH workshop on diagnostic devices for detecting antimicrobial susceptibility & resistance
- 4-8 Oct 2017 (San Diego): IDWeek
- 29 Oct-1 Nov 2017 (Santa Fe): Keystone Symposium – Antimicrobials and Resistance: Opportunities and Challenges
- 24-27 Nov 2017 (Taipei): 30th International Congress of Chemotherapy & Infection (ICC)
- 12-14 Feb 2018 (Baltimore): ASM Biothreats Conference
- 11-16 Mar 2018 (Ventura Beach): Gordon Research Conference on Antibacterial Discovery
- 21-24 Apr 2018 (Madrid): ECCMID
- 7-11 Jun 2018 (Atlanta): ASM Microbe
- 22-27 Jul 2018 (Bryant University, Smithfield, RI): Gordon Research Conference on Drug Resistance for Cancer, Infectious Disease and Agriculture
