Dear All: Two things today. Unrelated, but both relevant!
WHO’s GLASS: WHO’s Global Antimicrobial Resistance Surveillance System (GLASS) was created during October 2015 and seeks to obtain coordinated and consistent global estimates on resistance rates. In a major report released on 29 Jan 2018, GLASS now provides provides official national AMR data for the period 2016-17 from 40 countries based on testing of 507,746 isolates of Acinetobacter spp., Escherichia coli, Klebsiella pneumoniae, Neisseria gonorrhoeae, Salmonella spp., Shigella spp., Staphylococcus aureus, and Streptococcus pneumoniae. Participating countries span the globe:
The good news is that GLASS exists and that countries are making progress on creating National Coordination Centres (NCCs), National Reference Laboratories (NRLs), and sentinel surveillance sites. Progress is (no surprise) somewhat uneven, but the real point is that progress is happening.
The bad news is that substantial rates of resistance are seen world-wide. There is no single best way to summarize this, but here is an example of a resistance rate summary that you can find in the report (x-axis is % non-susceptible). Note the nearly 25% rate of imipenem resistance!
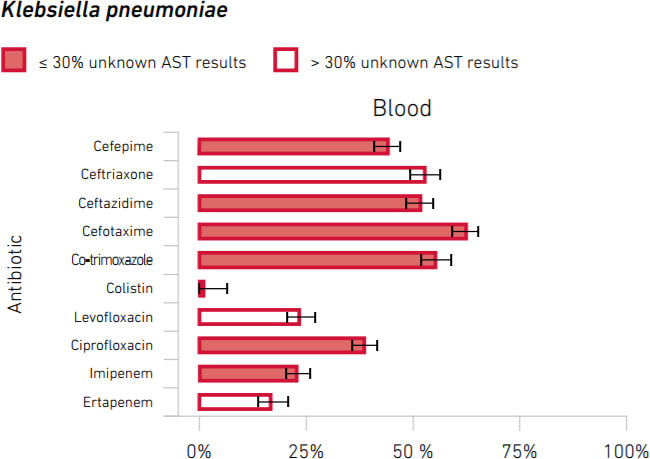
Scary!
FDA & PDUFA VI: FDA posted a small business-focused analysis of PDUFA VI back in November 2017 that I only recently learned about. As a reminder, the Prescription Drug User Fee Act (PDUFA) has been an evolving legislative series by which FDA collects fees that it then uses to make the drug review process more efficient while not compromising drug safety. There are many changes in PDUFA VI (please see the full summary here), but these are notable to my eye:
- Early Consultation on the Use of New Surrogate Endpoints: Early consultation between the FDA and sponsors can now occur when the sponsor intends to use a biomarker as a new surrogate endpoint that has never been previously used as the primary basis for product approval in the proposed context of use.
- Advancing Development of Drugs for Rare Diseases: CDER’s Rare Disease Program staff will provide their expertise on approaches to studying and reviewing such drugs, continue to foster collaborations in the development of tools and data to support rare disease drug development, and facilitate interactions to increase awareness of FDA programs and engagement of patients in the decision-making process.
- Advancing the Use of Complex Innovative Trial Designs and Model Informed Drug Development: FDA will conduct activities to facilitate the development and application of exposure-based, biological, and statistical models derived from preclinical and clinical data sources.
I hope you’ll quickly see why these are relevant to antimicobial agents: we’ve mused on surrogate endpoints (but time courses for major infections are so quick that it’s generally better to just get a real endpoint), we’ve discussed at length the problem of rare species (see materials from the 13 April 2017 AMDAC plus my blog on same), and we debated the question of alternative trial designs during the 18-19 July 2016 workshop discussion of hypothetical Drug X-1.
And, this sheds some light on the recently announced Duke-Margolis 1-day workshop on inclusion and exclusion criteria in clinical trials on 16 April 2018 as part of a cooperative agreement with FDA (corresponding FR notice) which will “discuss a variety of topics related to eligibility criteria in clinical trials and their potential impact on patient access to investigational drugs, and how to facilitate the enrollment of a diverse patient population.”
It all makes your head spin! Although we don’t know the pace with which all this will happen, it certainly reminds me of Picabia’s comment that “Our heads are round so our thoughts can change direction.”
Buckle up! –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://13.43.35.2/blog/
Upcoming meetings of interest to the AMR community:
- 12-14 Feb 2018 (Baltimore): ASM Biothreats Conference
- 28 Feb 2018 (Brussels): Deadline for responses to IMI2 Call 13 (Topic 3) on AMR diagnostics
- 2 Mar 2016 (Berlin): BEAM-sponsored 1-day “Big Drugs for Bad Bugs” conference
- 11-16 Mar 2018 (Ventura Beach): Gordon Research Conference on Antibacterial Discovery
- 12-13 Mar 2018 (London): BSAC’s Spring Conference
- 27-29 Mar 2018 (Cardiff, UK): BSAC-sponsored 3-day residential Workshop on Susceptibility Testing
- 16 Apr 2018 (Washington): Duke-Margolis & FDA event: “Evaluating Inclusion and Exclusion Criteria in Clinical Trials”
- 21-24 Apr 2018 (Madrid): ECCMID
- [NEW] 4-7 Jun 2018 (Boston): BIO (multiple AMR-focused sessions are expected)
- 7-11 Jun 2018 (Atlanta): ASM Microbe
- 22-27 Jul 2018 (Bryant University, Smithfield, RI): Gordon Research Conference on Drug Resistance for Cancer, Infectious Disease and Agriculture
- [Don’t miss this one] 4-7 Sep 2018 ESCMID-ASM Conference (#3) on Drug Development for AMR (Lisbon, Portugal) (initial flyer)
- 6-14 Oct 2018 International Course on Antibiotics and Resistance (ICARe, Les Pensières, Annecy, France)