Dear All:
Given our collective interest in finding oral antibiotics, a recent article in J Med Chem on the evolution of our understanding of Lipinski’s Rule of 5 (Ro5) prompts me today to share it along with a recent webinar and links to both Lipinski’s original paper as well as his 2016 commentary:
- The original paper: Lipinski, C. A., F. Lombardo, B. W. Dominy and P. J. Feeney (2001). “Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings.” Adv Drug Deliv Rev 46(1-3): 3-26: https://www.sciencedirect.com/science/article/pii/S0169409X00001290
- A 2016 commentary by Lipinski: Lipinski, C. A. (2016). “Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions.” Adv Drug Deliv Rev 101: 34-41: https://www.sciencedirect.com/science/article/pii/S0169409X16301399
- A September 2018 CDD Vault webinar with Lipinski: “A Discussion with Chris Lipinski and Barry Bunin: Recorded Webinar”
- A very recent commentary: Shultz, M. D. (2018). “Two Decades under the Influence of the Rule of Five and the Changing Properties of Approved Oral Drugs.” J Med Chem: https://pubs.acs.org/doi/10.1021/acs.jmedchem.8b00686 (see copy of abstract below my signature)
I think that everyone in the drug discovery process should have at least a passing familiarity with these ideas and their subsequent evolution. In brief, the original Ro5 states that a given compound is likely to have poor absorption and/or permeation if:
- There are more than 5 H-bond donors (expressed as the sum of OHs and NHs)
- The MWT is over 500
- The Log P is over 5 (or MLogP is over 4.15)
- There are more than 10 H-bond acceptors (expressed as the sum of Ns and Os)
- Compound classes that are substrates for biological transporters are exceptions to the rule
Note that the fifth rule declares that there are exceptions to the first four rules. Importantly for the AMR community, it turns out that antibiotics are frequently the source of those exceptions! This excerpt from Lipinski 2001 is worth a careful read (line breaks and emphasis added for ease of reading):
- “The ‘rule of 5’ is based on a distribution of calculated properties among several thousand drugs. Therefore by definition, some drugs will lie outside the parameter cutoffs in the rule.
- Interestingly, only a small number of therapeutic categories account for most of the USAN drugs with properties falling outside our parameter cutoffs. These orally active therapeutic classes outside the ‘rule of 5’ are: antibiotics, antifungals, vitamins and cardiac glycosides.
- We suggest that these few therapeutic classes contain orally active drugs that violate the ‘rule of 5’ because members of these classes have structural features that allow the drugs to act as substrates for naturally occurring transporters. When the ‘rule of 5’ is modified to exclude these few drug categories only a very few exceptions can be found.
- For example, among the NCEs between 1990 and 1993 falling outside the double cutoffs in ‘the rule of 5’, there were nine non-orally active drugs and the only orally active compounds outside the double cutoffs were seven antibiotics.“
The exceptions to the Ro5 become especially important when you realize that many antibiotics are natural products (NPs) and that “Most of the favorable exceptions to Ro5 occur among NPs,” as stated in Lipinski 2016. While there may be other drawbacks to NPs (most notably, it can be challenging to produce more than a limited number of derivatives of the starting molecule), violating the Ro5 is not necessarily a dead-end. I think this is relevant given the work by NCI to make available a very large natural product library — I do hope we see some AMR-focused efforts to screen this library.
Shultz’s 2018 commentary (see abstract just below my signature) goes even further to argue that the core rules (and especially MW) may have other weaknesses. I think that these further details are probably more than those of us who are not medicinal chemists (that’s me!) need to know, but overall I thought this screenshot from the CDD Vault webinar brought everything together nicely:
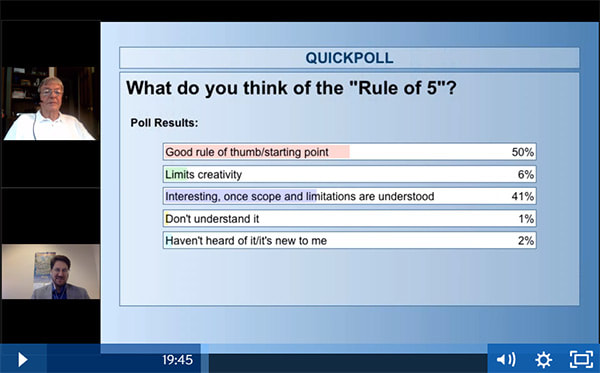
In summary, all this suggests there is potential for happy drug hunting both inside and outside of Lipinski space!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://13.43.35.2/blog/
Abstract from Shultz’s 2018 paper (line breaks added for ease of reading):
- Two decades have passed since the rule of five ushered in the concept of “drug-like” properties. Attempts to quantify, correlate, and categorize molecules based on Ro5 parameters evolved into the introduction of efficiency metrics with far reaching consequences in decision making by industry leaders and scientists seeking to discover new medicines.
- Examination of oral drug parameters approved before and after the original Ro5 analysis demonstrates that some parameters such as clogP and HBD remained constant while the cutoffs for parameters such as molecular weight and HBA have increased substantially over the past 20 years.
- The time dependent increase in the molecular weight of oral drugs during the past 20 years provides compelling evidence to disprove the hypothesis that molecular weight is a “drug-like” property.
- This analysis does not validate parameters that have not changed as being “drug-like” but instead calls into question the entire hypothesis that “drug-like” properties exist.
Opportunities of interest for the AMR community
- 11 Oct 2018 deadline: Novo’s REPAIR Impact fund has re-opened for proposals during the window 4 Sep – 11 Oct. Read more and apply here.
- 24 Oct 2018 deadline: IMI AMR Accelerator programme Pillar A within IMI Call 15: Capability-building network to manage the whole accelerator and strengthen AMR science. This is a two-stage call, with letter of intent from applicants expected on 24 Oct 2018.
- 24 Oct 2018 deadline: IMI AMR Accelerator programme Pillar B: Tuberculosis drug development network within IMI Call 15: Tuberculosis drug development network to collaboratively progress TB compounds and validate new tools for TB drug development. This is a two-stage call, with letter of intent from applicants expected on 24 Oct 2018.
- 24 Oct 2018 deadline: IMI Call 16: A series of individual programs where a single EFPIA partner works with a consortium to progress compounds for for TB, non-tuberculous mycobacteria, and Gram-negatives. This is a one-stage call, with full proposal from the EFPIA and applicant consortium expected on 24 Oct 2018.
Upcoming meetings of interest to the AMR community:
- 23 Oct 2018 (online webinar): Introduction to Pew’s SPARK platform (Shared Platform for Antibiotic Research and Knowledge)
- 23 Oct 2018 (New York City): New York Academy of Sciences workshop entitled “New Therapeutic Strategies to Combat Antibacterial Resistance“
- 26 Oct 2018 (London): EMA information day for SMEs: “Regulatory toolbox for medicines and combined devices developers”. Here is the current agenda. Webcast will be available. More details from sme@ema.europa.eu.
- 29-30 Oct 2018 (Washington): BARDA Industry Days, a 2-day conference on countermeasure development for the US Government
- 7-9 Nov 2018 (Seville, Spain): Better Methods for Clinical Studies in Infectious Diseases and Clinical Microbiology: A Hands-on Workshop
- 8 Nov 2018 (Alderley Park, UK): Bionow’s 1-day Bioinfect conference
- [NEW] 12-18 Nov 2018 (everywhere): WHO (and US CDC) Antibiotic Awareness Week. Events now being planned … see the WHO and CDC links for ideas, fact sheets, and graphical materials.
- 13 Nov 2018 (London): All-Parties Parliamentary Working Group on AMR meeting. Online materials here.
- 16 Nov 2018 (Berlin): Max Planck Institute for Innovation and Competition is organizing a conference on life sciences and innovation. Online materials here.
- 21 Nov 2018 (London): NICE- & APBI-sponsored masterclass: “Using non randomised data to estimate treatment effects in NICE submissions”. Details here.
- 29-30 Nov 2018 (Birmingham, UK): BSAC (British Society Antimicrobial Chemotherapy): Antibiotic Resistance Mechanisms Workshop for Researchers
- 7 Dec 2018 (Boston, MA): BAARN, Boston Area Antimicrobial Resistance Network 2018 symposium, 8:30am to 7pm at The Starr Center (185 Cambridge Street, Boston, MA). This is an excellent networking opportunity, especially for those based in the Boston area. Details not yet online.
- [NEW] 15 Jan 2018 (London): BSAC’s Antimicrobial Chemotherapy Conference 2019: “An ABC for everyone involved in developing new antimicrobials.” Details here.
- 14-15 Mar 2019 (Berlin): BEAM– and ND4BB-ENABLE-sponsored Berlin Conference on Novel Antimicrobials and AMR Diagnostics. Details here.
- 21-22 Mar 2019 (Birmingham, UK): BSAC Spring Conference.