Dear All:
Just released is a substantial report commissioned by Wellcome Trust entitled “Vaccines to tackle drug-resistant infections: An evaluation of R&D opportunities.” The full .pdf is here. The associated website is worth exploring. In particular, there is an interactive tool that allows you to explore the parameters used to calculate relative priorities.
As you probably already know, vaccines are an important part of our multi-pronged approach needed to tackle AMR. As the potential of vaccines is felt to be underutilized, this report was created to provide evidence to guide research priorities, policy focus and investment decisions for funders such as Gavi, the Global Vaccines Alliance.
To that end, the report considers the WHO priority pathogen list and then ranks the value of vaccine development for each by considering
- The potential health impact of a vaccine for that pathogen,
- The probability of R&D success, and
- The probability of vaccine uptake.
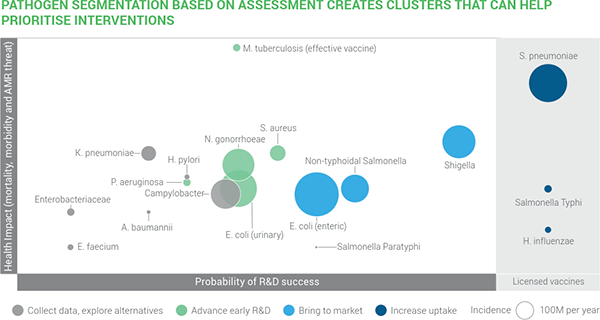
From a developer’s standpoint, these two graphics tell the story. In the first, we have 4 things displayed simultaneously:
- Y-axis: Impact
- Probability of R&D success
- Size of bubble: Incidence/year
- Color: Recommendation of the report on appropriate action
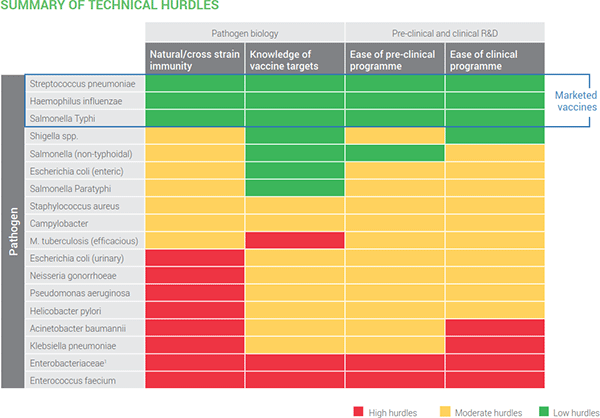
Second, we have this summary of technical hurdles. I think its color scheme is self-explanatory:
Vaccines have much to offer in our efforts to combat AMR and this report is a useful roadmap for both researchers and public health professionals.
As it takes on average 14.3 years (see page 14 of the report) to develop a new vaccine, the time to start is now. And as with antibiotics, we also have to think about commercial return. Vaccines cost just as much to develop as a new drug and typically have costs for establishing a manufacturing and supply chain that are much higher than for traditional antibiotics.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://13.43.35.2/blog/
Opportunities of interest for the AMR community
- 11 Oct 2018 deadline: Novo’s REPAIR Impact fund has re-opened for proposals during the window 4 Sep – 11 Oct. Read more and apply here.
- 24 Oct 2018 deadline: IMI AMR Accelerator programme Pillar A within IMI Call 15: Capability-building network to manage the whole accelerator and strengthen AMR science. This is a two-stage call, with letter of intent from applicants expected on 24 Oct 2018.
- 24 Oct 2018 deadline: IMI AMR Accelerator programme Pillar B: Tuberculosis drug development network within IMI Call 15: Tuberculosis drug development network to collaboratively progress TB compounds and validate new tools for TB drug development. This is a two-stage call, with letter of intent from applicants expected on 24 Oct 2018.
- 24 Oct 2018 deadline: IMI Call 16: A series of individual programs where a single EFPIA partner works with a consortium to progress compounds for for TB, non-tuberculous mycobacteria, and Gram-negatives. This is a one-stage call, with full proposal from the EFPIA and applicant consortium expected on 24 Oct 2018.
Upcoming meetings of interest to the AMR community:
- 23 Oct 2018 (online webinar): Introduction to Pew’s SPARK platform (Shared Platform for Antibiotic Research and Knowledge)
- 23 Oct 2018 (New York City): New York Academy of Sciences workshop entitled “New Therapeutic Strategies to Combat Antibacterial Resistance“
- 26 Oct 2018 (London): EMA information day for SMEs: “Regulatory toolbox for medicines and combined devices developers”. Here is the current agenda. Webcast will be available. More details from sme@ema.europa.eu.
- 29-30 Oct 2018 (Washington): BARDA Industry Days, a 2-day conference on countermeasure development for the US Government
- 7-9 Nov 2018 (Seville, Spain): Better Methods for Clinical Studies in Infectious Diseases and Clinical Microbiology: A Hands-on Workshop
- 8 Nov 2018 (Alderley Park, UK): Bionow’s 1-day Bioinfect conference
- [NEW] 12-13 Nov 2018 (London): Joint SCI– and Royal Society of Chemistry-sponsored 2nd annual Symposium on Antimicrobial Discovery. Online materials here.
- 12-18 Nov 2018 (everywhere): WHO (and US CDC) Antibiotic Awareness Week. Events now being planned … see the WHO and CDC links for ideas, fact sheets, and graphical materials.
- 13 Nov 2018 (London): All-Parties Parliamentary Working Group on AMR meeting. Online materials here.
- 16 Nov 2018 (Berlin): Max Planck Institute for Innovation and Competition is organizing a conference on life sciences and innovation. Online materials here.
- 21 Nov 2018 (London): NICE- & APBI-sponsored masterclass: “Using non randomised data to estimate treatment effects in NICE submissions”. Details here.
- 29-30 Nov 2018 (Birmingham, UK): BSAC (British Society Antimicrobial Chemotherapy): Antibiotic Resistance Mechanisms Workshop for Researchers
- 7 Dec 2018 (Boston, MA): BAARN, Boston Area Antimicrobial Resistance Network 2018 symposium, 8:30am to 7pm at The Starr Center (185 Cambridge Street, Boston, MA). This is an excellent networking opportunity, especially for those based in the Boston area. Details not yet online.
- 15 Jan 2018 (London): BSAC’s Antimicrobial Chemotherapy Conference 2019: “An ABC for everyone involved in developing new antimicrobials.” Details here.
- 14-15 Mar 2019 (Berlin): BEAM– and ND4BB-ENABLE-sponsored Berlin Conference on Novel Antimicrobials and AMR Diagnostics. Details here.
- 21-22 Mar 2019 (Birmingham, UK): BSAC Spring Conference.