Dear All (wonkish note alert … top-up your coffee and settle in!),
Today we have a tour of a recent paper in Clinical Infectious Diseases in which FDA presents a very instructive analysis of endpoints used in recent CABP (Community-Acquired Bacterial Pneumonia) trials and how data such as these might permit further convergence of global regulatory requirements.
As is often the case, a bit of context will be helpful. Here’s the reading list that I’ll use for our tour:
- FDA’s recent CABP paper (https://doi.org/10.1093/cid/ciaa860): Bart SM, Nambiar S, Gopinath R, Rubin D, Farley JJ. Concordance of early and late endpoints for community-acquired bacterial pneumonia trials. Clinical Infectious Diseases. 2020.
- An editorial on the CABP paper by George Talbot (https://doi.org/10.1093/cid/ciaa853): Talbot GH. Understanding Early and Late Endpoints in Registrational Trials of Community-acquired Bacterial Pneumonia. Clinical Infectious Diseases. 2020.
- An analysis of ABSSSI (skin infection) trial data that parallels the FDA CABP analysis (link): Nathwani D, Corey R, Das AF, Sandison T, De Anda C, Prokocimer P. Early Clinical Response as a Predictor of Late Treatment Success in Patients With Acute Bacterial Skin and Skin Structure Infections: Retrospective Analysis of 2 Randomized Controlled Trials. Clin Infect Dis. 2017;64(2):214-7.
- An editorial on ABSSSI paper by George Talbot (link): Talbot GH. The Early Clinical Response Endpoint: Great Timing by the Food and Drug Administration? Clin Infect Dis. 2017;64(2):218-20.
- And if you really want to do a deep dive into the early/late endpoint idea and current perspectives on trial designs, see further this group of papers:
- Read this one if nothing else: An integrated summary of the work to date on endpoints for CABP, ABSSSI, and HABP-VABP (link): Talbot GH et al. Developing Outcomes Assessments as Endpoints for Registrational Clinical Trials of Antibacterial Drugs: 2015 Update From the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis. 2016;62(5):603-7.
- The foundational paper on endpoints for CABP and ABSSSI (link): Talbot GH et al. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: Update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis. 2012;55(8):1114-21.
- A survey of the logic of non-inferiority trial designs and how endpoints are used in each (link): Rex JH et al. Progress in the fight against multidrug-resistant bacteria 2005-2016: Modern non-inferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin Infect Dis. 2017;65:141-6.
- A bit tangential, but this paper summarizes the data that underpin the all-cause mortality endpoint used for HABP-VABP (link): Talbot GH et al. Evidence-Based Study Design for Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia. J Infect Dis. 2019;219(10):1536-44.
—-
First stop: The new paper from FDA (link) shows strong concordance of early and late endpoints for CABP. For reasons discussed at length in the deep dive papers (if you do not know the back story, I strongly encourage you to read at least the first of those papers), George Talbot led a group of us during 2010-2012 on a project that validated both (i) a well-defined & reliable endpoint as well as (ii) a time for that endpoint that (iii) could be tied to high quality data showing the treatment effect of antibiotics relative to no antibiotics for both CABP and ABSSSI.
For CABP, progressive improvement in 4 symptoms (cough, dyspnea, chest pain, and sputum production) during the first 4 days on therapy was found to be a suitable measure. These symptoms are thus the basis of the Early Clinical Response (ECR) measured at 3-5 days after starting therapy that FDA uses as the basis for its analysis of trials of new agents for CABP.
Importantly, FDA uses ECR at days 3-5 because (i) data from the early antibiotic era suggest the greatest treatment effect is observed in this window, and (ii) those same data provide a robust justification for the statistical underpinnings of a non-inferiority trial (for more on this, see deep-dive paper #3, link). However, ECR at days 3-5 is also somewhat counter-intuitive as (i) the patient is not finished with therapy, (ii) late complications (e.g., empyema) won’t manifest by this point, and (iii) what really matters to the patient is ultimately completing the full course of therapy and returning to full health (for more on this, see Talbot’s discussion in paper #4, link.)
In part because of these concerns, other agencies (EMA, PMDA) use an overall clinical outcome at a test-of-cure (TOC) visit 5-10 days after the end of therapy. And, FDA also analyzes the late overall outcomes and includes those data in product labeling: for an example of this, see Section 14.1 of the omadacycline label (link).
As a consequence of these regulatory differences, a modern global trial of a new agent for CABP will include measures of both of these endpoints. As it makes good biological sense that the two endpoints would align, the presumption was that the different measures would give similar demonstrations of efficacy when these paired endpoints entered global usage.
And, the message of Bart et al. is that this is indeed true: the two endpoints are very concordant in their analysis of data on 4,645 patients from 6 recent trials. Here’s the key table:
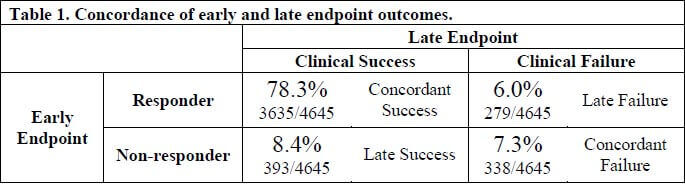
As you can see, 85.6% (78.3% + 7.3%) of patients had identical early and late endpoint outcomes and likewise, similar numbers (8.4% and 6.0%) had discordant outcomes. The positive predictive value (PPV) of early success was high at 93% but interestingly the negative predictive value (NPV) of early failure was only 46% … pretty much a coin toss as to whether you’d go on to have an overall successful late response after not having responded in the first few days.
FDA analyzed the subjects with early success + late failure discordance (6.0%) in detail and found that prior antibacterial therapy, C. pneumoniae infection, S. aureus infection, and severe chest pain predicted this outcome. Discordance due to prior antibacterial therapy is especially interesting as it suggests that even a single prior dose of a short-acting antibiotic (the limit for these trials) could produce a misleading early success. (Aside: the potential for prior antibiotics to confuse trial results is a big point of concern … see Pertel et al. CID 2008;46(8):1142-51, link, for the best-studied example of this effect.)
There’s not a lot of discussion of the opposite discordance (early failure + late success), but one can speculate that patients not doing well at Day 4 may have had their therapy changed.
—
Second stop: OK, so what does this mean for drug developers? Well, FDA notes this insight could set us up for global regulatory convergence for CABP trials. As noted above, developers currently need different statistical analysis plans for different agencies. Could we one day collapse them into a common set of endpoints? And if non-inferiority margins could be made the same, we’d arrive at a common design for global trials.
Further, the 3rd and 4th papers in the reading list suggest this might also be possible for ABSSSI (Acute Bacterial Skin and Skin Structure Infections) — I’m not going to review the data at length, but FDA uses an early response endpoint of lesion size after 48h for ABSSSI that was shown in a secondary analysis of the two tedizolid registrational trials to have good concordance with a standard late clinical response endpoint.
—
Third stop: Other insights from these papers. As an aside, there is an interesting observation about use of mortality as an endpoint in CABP discussed both in the Bart paper and in Talbot’s editorial. This approach is sometimes suggested as (i) 30-day CABP mortality rates for large surveys typically are > 10% (e.g., Peyrani 2020, link or Garcia-Vidal 2008, link), (ii) mortality has unequivocal clinical relevance, and (iii) Day 28 all-cause mortality is already used for HABP/VABP (Hospital- and Ventilator-Associated Bacterial Pneumonia).
In conclusion, the analysis by Bart et al. shows definitively that mortality rates in CABP trial participants simply do not reach this level and that mortality will never be a practical endpoint. Across the 4,645 patients in the pooled trials, the 976 with class IV PORT baseline scores (PORT is a severity score that runs from I to V) had a mortality of 3.7%. This is so low that it would take 3,000 PORT IV patients to run a meaningful trial. And although the PORT V patients had a mortality of 14.3%, there were only 28 PORT V patients enrolled across the entire set of six trials … it would never be feasible to run a study only in that subgroup of patients.
—
Many thanks to our colleagues at FDA for providing this instructive analysis. And while I’m at it, let me again thank George Talbot for his many years of leadership of the various projects that underpin all this work.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
PS: Despite the difficulties of this area, our community continues to grow. One measure of this is that the subscriber list for this newsletter has just passed 2,000 readers all over the world: the map shows a guess about the location of your fellow readers (darker = more). Many thanks for being part of this journey!
Current funding opportunities:
- Novo REPAIR Impact Fund is open for global applications through 31 Jul 2020. Go here for current details.
- 2020 funding rounds for CARB-X have not been announced.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes funders and projects by geography, stage, and more.
Upcoming meetings of interest to the AMR community:
- 9 Jul 2020 (online, 09:00-10:30 CEST): GARDP REVIVE webinar. Title: “The challenges and opportunities for antimicrobial R&D in low- and middle-income countries – India case study.” Speaker: Anand Anandkumar and Kamini Walla. Go here to register.
- 17 Jul-2 Aug 2020 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 27 Jul-31 Jul 2020 (online): Small World Initiative Instructor Training Workshop – training for undergraduate professors and high school teachers in wet lab techniques, parallel curricula, & pedagogical instruction to engage students in the hunt to find new antibiotics in soil (also covering distancing learning options). Go here to register.
- 4 Aug 2020 (Silver Spring): FDA workshop entitled “Development Considerations of Antifungal Drugs to Address Unmet Medical Need.” Go here to register.
- 5 Aug 2020 (Silver Spring): FDA workshop entitled “Developing Antifungal Drugs for the Treatment of Coccidioidomycosis (Valley Fever) Infection.” Go here to register.
- September 2020. University of Sheffield (UK). Applications are being taken for a new 1-year (full-time) or 2-year (part-time) Masters of Science course in Antimicrobial Resistance. The program runs annually from September and covers microbiology, clinical practice and policy. The course webpage is here.
- 9-10 Sep 2020 (Washington, DC): US PACCARB public meeting. Go here for details.
- 26-29 Oct 2020 (online meeting), Annual ESPID meeting (European Society for Pediatric ID, #38)
- [NEW] 27 Oct 2020 (online meeting), BARDA Industry Day, a discussion of U.S. Government medical countermeasure priorities. Mark your calendar now and watch this website for details.
- 10-13 Apr 2021 (Vienna): Annual ECCMID meeting (#31)
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 3-7 Jun 2021 (Anaheim), ASM Microbe 2021. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students.
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.