Dear All (and with thanks to Kevin Outterson for co-authoring this newsletter),
Just out in CID is a paper in which FDA analyzes trends in antibacterial development from 1980-2019. To fully appreciate this paper, you need to look both at it and three other papers:
- FDA’s paper (https://doi.org/10.1093/cid/ciaa859): Dheman N, Mahoney N, Cox EM, Farley JJ, Amini T, Lanthier ML. An Analysis of Antibacterial Drug Development Trends in the US, 1980 – 2019. Clinical Infectious Diseases. 2020.
- An editorial on the paper by Kevin and me (https://doi.org/10.1093/cid/ciaa852): Rex JH, Outterson K. Antibacterial R&D at a crossroads: We’ve pushed as hard as we can … now we need to start pulling! Clinical Infectious Diseases. 2020.
- A 2014 paper that foreshadowed FDA’s findings (link): Kinch MS, Patridge E, Plummer M, Hoyer D. An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov Today. 2014;19(9):1283-7.
- A separate recent analysis of antibacterial approvals (link): Darrow JJ, Najafzadeh M, Stefanini K, Kesselheim AS. Regulatory approval characteristics of antimicrobial versus non-antimicrobial products, 1984-2018: an evaluation of Food and Drug Administration flexibilities. The Lancet Infectious Diseases. 2020;20(7):e159-e64.
Starting with FDA’s paper (link), the concept was to identify every initial antibacterial IND (that is, every Investigational New Drug application received by FDA as the first step in clinical studies) from the period 1980-2019 and to then use FDA’s unparalleled perspective on drug development to see what has happened by decade. You’ll want to read the paper yourself, but let’s look at the paired graphs in the paper’s Figure 2 for a very powerful story:
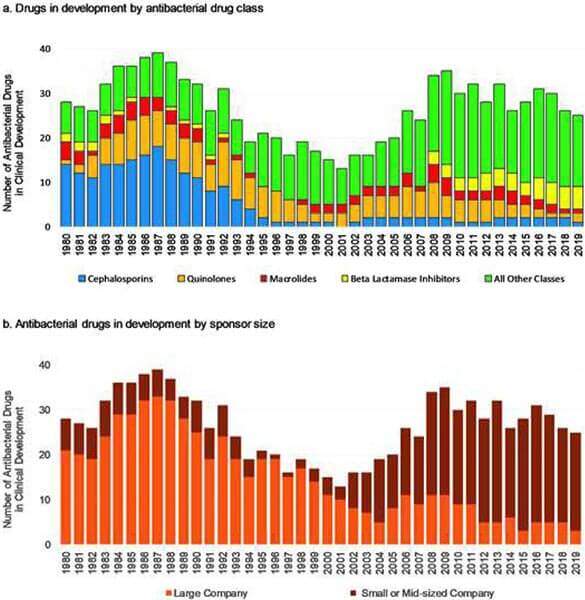
What we have in the upper graph is the # of antibacterials in development during each of the last 40 years (key point: the analysis was limited to systemic antibacterials … agents for C. difficile, H. pylori, and M. tuberculosis were excluded). Note how antbacterial R&D began to again gather momentum after the lull around 2000 … but then note the steam seems to going out of the efforts: As of 31 Dec 2019, only 25 antibacterials were in development, a number below that of 1980.
And there’s yet further bad news: the average time from IND to approval rose from 6 to 8.3 years (and trends in more recent data suggest that average time will ultimately exceed 9 years), the success rates fell from 40% to 23%, and the number of large companies involved fell steadily: Finally, only 3 large-company sponsored INDs were in active development as of 31 December 2019, the lowest level observed over the entire 40-year survey window (lower graph).
—
As noted above, these findings were foreshadowed in 2014 by the paper by Kinch (link) … and none of this will come as a surprise to those of us working in this space. Thus, our accompanying editorial (link) surveys both the challenges of this space and our evolving thinking:
- Finding compelling new antibacterial agents is very, very hard
- Both the science and the economics are challenging (link to a relevant newsletter)
- Calls for action have been raised and have led to some concrete actions
- We’ve seen IDSA’s 10×20 initiative, ND4BB, CARB-X, and much more (link to a survey of incentive reports)
- But, company-crushing post-approval economic pressure on antibacterial development remains unabated
- The failures of Achaogen (newsletter), Melinta (newsletter), and Tetraphase (newsletter)…
- And (perhaps a surprise), there is hope that Worst of Times might be transformed into the Best of Times
- The UK Subscription Pilot (newsletter), the DISARM Act (newsletter), and more (Hint! Sign up here for that 9 July webinar on the new global AMR initiative)
—
And to complete our tour of the challenges of R&D, please also review Darrow 2020 (link). Based on external data on drugs approved during 1984-2018, these authors have compared the application of regulatory flexibility to antimicrobial vs. non-antimicrobial products. In a very important difference from the FDA analysis, the scope of this review includes all types of antimicrobials, not just antibacterials. These authors found that antimicrobial products as a whole:
- were more likely to benefit from priority review, fast-track designation, and accelerated approval;
- were less likely to have Orphan Drug Act designation; and
- had a median time from investigational new drug application to approval of 5·9 years vs 7·6 years for other drug classes.
But when you dig closely (see the paper’s Figure 1), you see that this seemingly favorable pattern is dominated by these advantages playing out mostly for antivirals, antiprotozoals, antimycobacterials, and antifungals. Antibacterials, on the other hand, fall behind these other groups in their use of every type of expedited designation: Orphan Drug, Priority Review, Fast Track, Accelerated Approval, and Breakthrough Therapy Designation.
—
OK, so what does all this mean? Well, antibacterials are hard. Really, REALLY hard. And, we need more of them. So, and to quote the title of our editorial: We’ve pushed as hard as we can … now we need to start pulling! It’s time to create some delinked incentives!
All best wishes, John & Kevin
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Kevin Outterson, JD, Professor of Law, Boston University & Executive Director, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of its funders) @koutterson
Current funding opportunities:
- Novo REPAIR Impact Fund is open for global applications through 31 Jul 2020. Go here for current details.
- 2020 funding rounds for CARB-X have not been announced.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes funders and projects by geography, stage, and more.
Upcoming meetings of interest to the AMR community:
- [NEW] 30 June (online, 11.00-16.30 EDT): US DARPA Proposer’s Day in support of a planned Broad Agency Announcement (BAA) for their HEALR Program seeking novel antimicrobials. Go here for more on HEALR and here to register for the webinar.
- 30 Jun 2020 (online, 17:00-18:30 CEST): GARDP REVIVE webinar. Title: “Clinical development of antimicrobials – Phase 1 development challenges.” Speaker: Markus Zeitlinger. Go here to register.
- 9 Jul 2020 (online, 09:00-10:30 CEST): GARDP REVIVE webinar. Title: “The challenges and opportunities for antimicrobial R&D in low- and middle-income countries – India case study.” Speaker: Anand Anandkumar and Kamini Walla. Go here to register.
- 17 Jul-2 Aug 2020 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 27 Jul-31 Jul 2020 (online): Small World Initiative Instructor Training Workshop – training for undergraduate professors and high school teachers in wet lab techniques, parallel curricula, & pedagogical instruction to engage students in the hunt to find new antibiotics in soil (also covering distancing learning options). Go here to register.
- 4 Aug 2020 (Silver Spring): FDA workshop entitled “Development Considerations of Antifungal Drugs to Address Unmet Medical Need.” Go here to register.
- 5 Aug 2020 (Silver Spring): FDA workshop entitled “Developing Antifungal Drugs for the Treatment of Coccidioidomycosis (Valley Fever) Infection.” Go here to register.
- September 2020. University of Sheffield (UK). Applications are being taken for a new 1-year (full-time) or 2-year (part-time) Masters of Science course in Antimicrobial Resistance. The program runs annually from September and covers microbiology, clinical practice and policy. The course webpage is here.
- 9-10 Sep 2020 (Washington, DC): US PACCARB public meeting. Go here for details.
- 26-29 Oct 2020 (online meeting), Annual ESPID meeting (European Society for Pediatric ID, #38)
- 10-13 Apr 2021 (Vienna): Annual ECCMID meeting (#31)
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 3-7 Jun 2021 (Anaheim), ASM Microbe 2021. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students.
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.