See also
- This related newsletter: 14 Jul 2022, entitled “WHO Vaccine Pipeline Review; CDC On Impact Of COVID On AMR.”
- This 20 Oct 2024 report from WHO: “Estimating the impact of vaccines in reducing antimicrobial resistance and antibiotic use: technical report”
- This 25 Apr 2024 paper in Lancet Global Health by Hausdorff and colleagues: “Facilitating the development of urgently required combination vaccines” DOI https://doi.org/10.1016/s2214-109x(24)00092-5
Dear All: As part of their Immunization Agenda 2030: A Global Strategy to Leave No One Behind, WHO have now published an AMR-focused action framework that summarizes ways we should seek to use vaccines both to reduce antibiotic use and to prevent the further emergence of antimicrobial resistance. Here are the links you need — I suggest you start with the first two, saving the others to explore as a deeper dive:
- The AMR-focused Action Framework that we’ll be discussing
- An overview paper on the Action Framework by Vekemans et al. in Clinical Infectious Diseases
- A very detailed implementation plan for the overall 2030 Immunization Agenda (all vaccines, not just AMR-focused)
- The website for Immunization Agenda 2030
The AMR-focused Action Framework has 3 core goals:
- Expanding Use: In brief, we need to use the vaccines we have!
- New Vaccines: In brief, a few new vaccines seem possible, but only a few
- Expanding Knowledge of Vaccine Impact: In brief, driving policy change requires data!
Before we set off on our tour, please note that we are discussing the elements of the Immunization Agenda 2030 that are focused on vaccines for the standard bacteria. New vaccines for tuberculosis and vaccines for viral infections are also important but are out of scope (but as we will note, deploying antiviral vaccines can impact AMR, so there is some overlap). With that context noted, let’s take the tour!

This goal is broken into 3 objectives: (i) Expanding use, (ii) Updating guidance documents (and the data behind them), and (iii) Communication / Education.
In a way, this is both the easiest and most challenging goal! On the easy side, the point is that we already have a lot of great vaccines: the pneumococcus (S. pneumoniae, the cause of pneumonia across all ages), Haemophilus influenza type B (causes meningitis in children), rotavirus, measles, and influenza are well established and need to be used more widely. A newcomer on the scene is a conjugate vaccine for typhoid that also needs to rolled into the global distribution network.
Using these vaccines impacts AMR In two ways. For the actual bacterial infections (e.g., pneumococcal pneumonia), it’s obvious that a pneumonia that doesn’t occur is a pneumonia that doesn’t need treatment. But what about measles? Well, the point here would be that an episode of measles can set you up to need antibiotics for a secondary infection. Stated differently, staying healthy overall and not having symptomatic episodes of viral or bacterial infections is a great way to reduce use of antibiotics!
So, why do I say that this is also the most challenging goal? Well, the difficulties with both distribution and acceptance of vaccines are well documented … developing public trust takes sustained effort. This is hard work without any sort of single easy answer.

The objectives for this goal are what you’d expect: (i) ensure adequate funding for needed/plausible R&D and (ii) streamline regulatory and policy processes to the maximum extent possible.
The funding challenges and solutions here are similar to those for antibacterial agents. The R&D is slow and expensive and we need push-pull mechanisms such as subscription models.
The question of needed/plausible R&D is a very deep one. There are lots of bacterial infections that could use a vaccine, but not all vaccines are equally plausible. The 2018 Wellcome Trust-funded reported entitled Vaccines to tackle drug resistant infections: An evaluation of R&D opportunities tackles the question of need vs. plausibility and proposes that we think of possible new vaccines in 3 categories: (i) Bring to Market (i.e., get going and do it!), (ii) Advance Early R&D (i.e., looks possible, lets engage fully), and (iii) Collect Data and Explore Alternatives (Hmm — not obvious we can ever get there so keeping exploring while looking in parallel for non-vaccine approaches). When you consider the world of possible bacterial vaccines through this lens, here’s what you get:
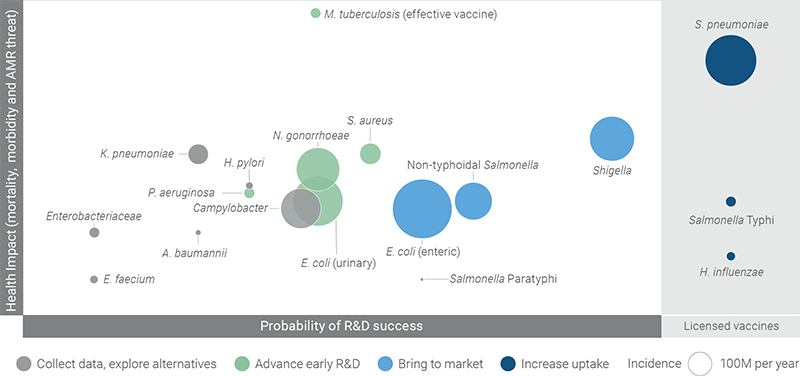
Looking closely, we can see that the graphic adds the category Increase Uptake for established vaccines, thus reflecting back to Goal 1. The Bring to Market group focuses on vaccines for E. coli and Shigella as causes of gut (enteric) infections. The Advance R&D group is diverse and includes Staphylococcus aureus, Neisseria gonorrhoeae, E. coli (but now for UTI), and P. aeruginosa. All other bacteria fall into the Collect Data/Explore Alternatives bucket.
What’s instructive here is that this review found that vaccines are most likely for a limited number of additional bacteria. We should keep trying, but it would be good to hedge our bets for the other bacteria with parallel efforts to prevent infections both via general public health measures (clean food, clean water!) and via developing new antibiotics.

This goal has two objectives that really focus on public policy: (i) Collect more/better data on the impact of vaccines and (ii) Develop estimates of the value of vaccines.
These are the sorts of data that move public policy — legislative / political change only happens when we can clearly articulate the “What does it cost? What do get for our money?” side of things to political and financial leaders.
Such data are especially important as prevention involves paying for things to NOT happen. It’s so easy to see what you get when you pay for a new bridge (and you can even name it for somebody) but the equivalent for children who did NOT develop infection X is simply much less tangible. Thus, we have to measure it and prove its value over and over. If antibiotics are the fire extinguishers of medicine, then vaccines are the building codes that prevent cities from burning down! (Aside: the linked article on the history of building codes is fascinating … even Hammurabi had something to say on this topic during his reign in 1955-1913 BCE!).
—
Great stuff! As the paper by Vekemans et al. concludes:
- “Health interventions and policies depend on public confidence.
- “Advocacy and targeted communication can contribute to increased knowledge and catalyze the action needed to better protect everyone against infections and curb the threat that AMR poses to individuals, societies, and global health.”
In short, this community has a role to play as advocates for vaccines. By keeping us healthy, vaccines can delay / avert the rise of AMR. That noted, many of our fellow citizens do not understand how vaccines work and each one of us can be a source of needed insight!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- Novo REPAIR Impact Fund will open its next global call on 1 April 2021. Go here for current details.
- NIAID’s 2021 Broad Agency Announcement for product development is entitled “Development of Medical Countermeasures for Biothreat Agents, Antimicrobial-Resistant Infections an Emerging Infectious Diseases” and is now live with a 24 May 2021 deadline. Research areas include Vaccines, Therapeutics, and Sequencing-Based Diagnostics.
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 20-22 April 2021 (online, 1-5p CET): JPIAMR-sponsored workshop entitled “Feeding the Antimicrobial Therapeutics Pipeline.” JPIAMR, the collaborative global effort of 28 countries (much of the EU and also Argentina, Canada, Egypt, Israel, Japan, Korea, South Africa, and Turkey), is organizing a mixture of keynote talks, abstract presentations, and discussion panels designed to encourage collaborative antibiotic R&D. Go here for more details and registration. Abstract deadline is March 17th 2021; no previous JPIAMR funding required.
- [NEW] 29 April 2021 (online, 5-6.30p CEST): GARDP-sponsored webinar entitled “AMR R&D efforts in the CMC and formulation arena: Do it right the first time!” Go here to register.
- 10-12 May 2021 (virtual): UK-focused Virtual AMR Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free 3-day virtual event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. It will be followed (COVID-willing!) by a face-to-face mission scheduled for 11-15 Oct 2021. Go here for more details.
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 24-29 May 2021 (online and in Geneva): ESPID 2021, the 39th Annual Meeting of the European Society for Paediatric Infectious Diseases. Save-the-date announcement is here, details to follow.
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 20-24 Jun 2021 (virtual, various times): World Microbe Forum sponsored by the American Society for Microbiology (ASM) and the Federation of European Microbiological Societies (FEMS). Go here for more details and to register.
- 27 Jun-2 Jul 2021 (Ventura, CA): Gordon Research Conference entitled “Antimicrobial Peptides”. Go here for details, go here for the linked 26-27 Jun Gordon Research Seminar that precedes it.
- 9-12 Jul 2021 (virtual): Annual ECCMID meeting (#31)
- [NEW] 26 Jul-30 Jul 2021 (online): Small World Initiative Instructor Training Workshop – training for undergraduate professors in the wet lab techniques, parallel curricula, & pedagogical instruction to engage students in the hunt to find new antibiotic-producing soil microbes. Go here to register.
- 14-29 Aug 2021 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 11-15 Oct 2021 (physical, somewhere in the UK): UK-focused Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. Go here for more details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students. Bonus feature: For obvious reasons, the course didn’t happen in 2020! But as a celebration of the course’s 5th year, a webinar version was held on 29 Oct 2020: go here to stream it.
- 25-28 Oct 2021 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.