See also
- This related newsletter: 29 Mar 2021, entitled “Vaccines to turn back the tide of antimicrobial resistance.”
- This 20 Oct 2024 report from WHO: “Estimating the impact of vaccines in reducing antimicrobial resistance and antibiotic use: technical report”
- This 25 Apr 2024 paper in Lancet Global Health by Hausdorff and colleagues: “Facilitating the development of urgently required combination vaccines” DOI https://doi.org/10.1016/s2214-109x(24)00092-5
Dear All, WHO continues to crank out those pipeline reviews … now we have one for bacterial vaccines! Go here for the press release and here for the report. The report considers data from 2010 forward and (interestingly) provides data on both active and failed projects. The review identified 94 active preclinical candidates and 61 projects in the clinic. The distribution of clinical projects is instructive:
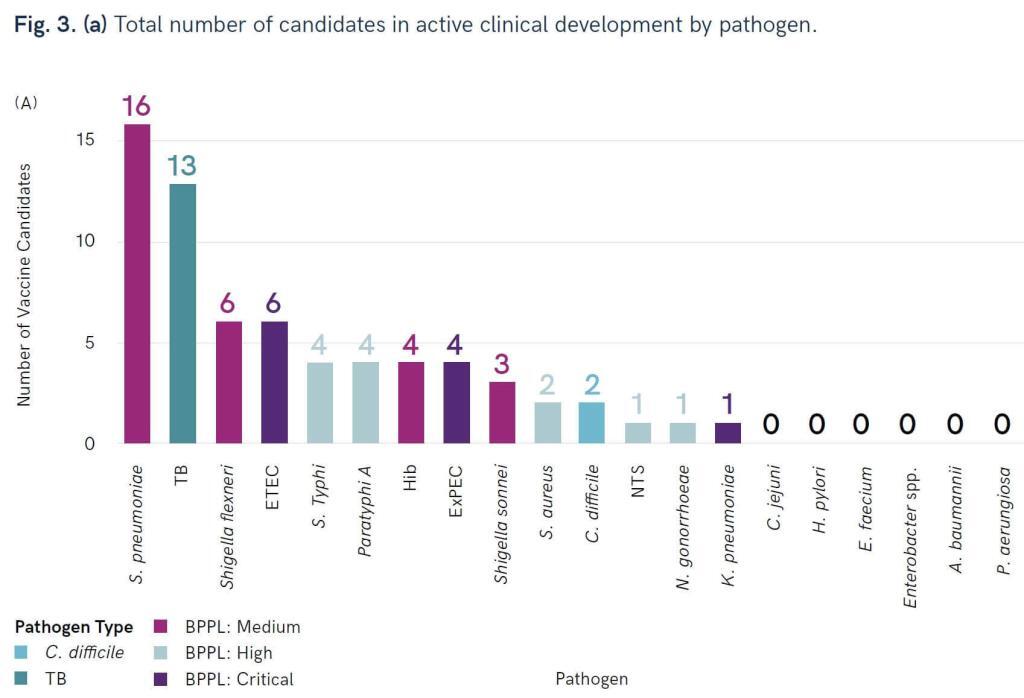
In the figure, BPPL = Bacterial Priority Pathogen List (go here if you need to see the current priority pathogen lists). You can thus see that we have reasonable coverage of Medium and High priority pathogens, but Critical pathogens are not well served.
Unfortunately, that gap in vaccine spectrum is unlikely to be fixed. As the report notes, “six pathogens from the WHO priority pathogens list (A. baumannii, P. aeruginosa, Enterobacter spp., E. faecium, S. aureus, and H. pylori) … were found to have low feasibility for vaccine development due to biological and other product development challenges.” Sad, but true! A real strength of the report is its discussion of the likelihood of success of the various types of projects; for more on feasibility issues, see also this 29 Mar 2021 newsletter on WHO’s Immunization Agenda 2030: A Global Strategy to Leave No One Behind.
—
CDC has just released a review on the impact of COVID-19 on AMR. Go here for the press release and here for the report. The key finding can be stated briefly: “resistant hospital-onset infections and deaths both increased at least 15% during the first year of the pandemic.” The results varied somewhat by pathogen (see table at the top of report page 6) with the sharpest increase being a 78% rise in the incidence of carbapenem-resistant Acinetobacter. Ugh!
Sadly, this result would seem predictable based on a prior CDC NHSN report (Weiner-Lastinger 2021, doi:10.1017/ice.2021.362) showing significant increases during 2020 in rates of central-line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated events, and MRSA bacteremia.
Many thanks to our colleagues at CDC for compiling and sharing these data! Now is the time for action at levels of the AMR ecosystem. Prevention, stewardship, new diagnostics, new therapeutics are needed! Let’s get the PASTEUR Act passed!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- The AMR Industry Alliance has announced their 2022 Stewardship Prize! The program offers 10,000 CHF to recognize established, innovative approaches to AMR stewardship in low- and middle-income countries (LMICs). The 2021 prize went to the Infectious Diseases Institute (IDI) in Kampala, Uganda for their best practices in diagnostic stewardship and for their patient awareness campaigns dedicated to decreasing inappropriate use of antibiotics in their specialist HIV clinic in Kampala. Applications for the 2022 prize are due August 31, 2022. Thinking in terms of stewardship, WHO have recently released a pair of courses through the OpenWHO platform:
- Course 1 provides training on the WHO Policy Guidance on Integrated Antimicrobial Stewardship Activities
- Course 2 focuses on the WHO practical toolkit for antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries
- The online training courses can be found on the dedicated channel: https://openwho.org/channels/amr
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is a newly launched early-stage funding vehicle. Details are still coming into focus, but per comments on 25 Aug 2021 at the BIOCOM conference, their goal is to support ~4 companies per year with about $250k/company. Contact details are on their website (https://www.incate.net/).
- New funding rounds from CARB-X are expected soon now that funding for the next 10 years has been announced! For the most current update, watch this 30-minute video from the June 2022 kick-off webinar.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- [NEW] 20 Jul 2022 (virtual. 11a-noon ET): Pfizer– and CQ Roll Call-sponsored webinar entitled “Superbugs: Policy Solutions to Tackle an Emerging Health Threat.” This webinar will feature Senators Todd Young (R-IN) and Michael Bennet (D-CO), the senate sponsors of the PASTEUR Act! Don’t miss it! Go here to register.
- 26 July 2022 (virtual, 10a-11.30a ET): REVIVE webinar entitled “New approaches for antibiotic discovery”. Go here for details.
- 24-27 July 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.
- 28-31 July 2022 (Singapore): 10th International Congress of Asia Pacific Society of Infection Control is a hybrid event for professionals in the Asia Pacific region. Go here for details and to register.
- 10 Aug 2022 (virtual, 11a-12.30p ET): REVIVE webinar entitled “Animal models to study the activity of antibiotics.” Go here for details.
- 10 Aug 2022 (virtual, 10a-4.30p ET): USDA ‘s APHIS (Animal and Plant Health Inspection Service) will host a public meeting on Antimicrobial Resistance. Co-hosted with USDA’s FSIS (Food Safety Inspection Service) and REE (Research, Education and Economics) mission area, the webinar will review lessons learned since the first AMR workshop 2012 as well as discuss plans for the future. Go here to register.
- 23 August 2022 (virtual, 11a-12.30p ET): REVIVE webinar entitled “The Challenges and options in developing antibiotic combinations.” Go here for details.
- 30 August 2022 (virtual, 8.30a-5.00p ET): Webinar sponsored by CDC and FDA entitled “Drug Development Considerations for the Prevention of Healthcare-Associated Infections.” This is very timely as developing products for prevention is surprisingly hard. As just one example, see this 20 Dec 2018 newsletter about a valiant (but failed, sadly) effort to develop a S. aureus vaccine. Go here for additional details and to register.
- 12-13 Sep 2022 (virtual, 9a-5p ET): This meeting of PACCARB is going to “identify key issues and critical policy gaps through a series of facilitated discussions examining a hypothetical large-scale disease outbreak scenario based on historic examples and estimates of future AMR outbreaks.” Sounds like pandemic wargaming (Center for Health Security; pre-COVID 19 May 2020 NPR article) to me! Go here for details.
- 20-24 Sep 2022 (New Delhi): 21st Congress of the International Society for Human and Animal Mycology (ISHAM). Go here for details.
- 4-7 Oct 2022 (Dublin, Ireland): The 2022 ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. This is an excellent meeting, especially for developers … and if you’ve missed it, the recordings from the 2021 meeting are online. Go here for details on the 2022 meeting.
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- [NEW] IDWeek has opened pipeline sessions for antibacterials, antifungals, and non-HIV, non-COVID antivirals as well as for diagnostics (no limitations). Go here to submit your abstract; this 14 July 2022 newsletter contains some additional details.
- 15-23 Oct 2022 (in person, residential, Les Pensières, Veyrier-du-Lac, France): The 6th edition of Patrice Courvalin’s fabulous ICARe residential training course covering all things AMR is on for 2022! This is a soup-to-nuts training in AMR: it is very intense, very detailed, and always gets rave reviews from attendees. Registration is open 21 Mar 2022 to 21 June 2022 and is limited, so book your slot as soon as you can. Go here for details.
- 19-23 Oct 2022 (Washington, DC): IDWeek 2022, the joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP). Go here for details.
- [NEW] 23 Oct 2022 (Cape Town, South Africa): Symposium entitled “Tackling AMR: How implementation research is vital in a One Health approach” that is sponsored by the AMR knowledge hub of TGHN (The Global Health Network). Go here for details.
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 17-20 Nov 2022 (Kuala Lumpur, Malaysia): The International Congress on Infectious Diseases will take place for the first time as a hybrid event. Go here for details.
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.