Addendum: This is the third in a 3-newsletters series on this topic. I need to do a specific newsletter on the challenges of vaccines but for now this is the place where I am accumulating relevant papers.
- Go here and here for the prior newsletters.
- 5 Feb 2023 addendum: A GSK Staphylococcus aureus vaccine candidate (GSK3878858A) was reported in GSK’s end-2024 pipeline update as “Not progressing following futility analysis.” This intriguing 4-component vaccine was being studied in a 632-subject Phase I/II study (NCT04420221). Sigh … a very tough area!
- 15 July 2023 addendum: The results of Pfizer’s STRIVE trial have been published as Hassanzadeh et al. in Clinical Infectious Diseases (2023;77:312-20, https://doi.org/10.1093/cid/ciad218).
- With the Hassanzadeh et al. paper, there is also an accompanying editorial (Proctor RA, https://doi.org/10.1093/cid/ciad217) that provides an excellent discussion of the complexity of the S. aureus-human immune system-human microbiome interaction and why this has made vaccine development so hard.
Dear All:
Per a press release today:
- Pfizer Inc. (NYSE:PFE) announced today that the Phase 2b trial STRIVE (STaphylococcus aureus SuRgical Inpatient Vaccine Efficacy) evaluating the company’s investigational Staphylococcus aureus (S. aureus) multi-antigen vaccine (PF-06290510) is being discontinued due to futility. This decision is based on a recommendation from an independent Data Monitoring Committee (DMC), composed of external experts, after conducting a pre-planned interim analysis.
- The DMC concluded from these data that the study reached futility, meaning that there is low statistical probability for the study to meet the pre-defined primary efficacy objective in adults undergoing elective spinal fusion surgery after completing a planned Phase 3 expansion of the study.
- A safety review by the DMC indicated that the investigational vaccine has been safe and well tolerated. STRIVE trial participants who are enrolled in the study will complete the study’s follow-up evaluations.
- Pfizer is evaluating next steps for the potential development of a S. aureus vaccine
I am so sorry to read this … I had thought this a very strong effort. Details are limited but as a “safety review by the DMC indicated that the investigational vaccine has been safe and well tolerated,” the problem must have been that it was not going to be possible show a reduced infection rate over placebo.
Recall that this vaccine was the subject of a 7 Nov 2017 FDA VRBPAC meeting during which questions about whether (and how) such a vaccine could be developed for general use (go here for the materials from that meeting). As a reminder,
- Pfizer identified elective open, posterior approach, multilevel, instrumented, spinal fusion surgery (read that carefully) might have a sufficiently high rate of post-operative infections (estimated at 1.44%) despite best prevention methods that the effect of a vaccine could be measured by studying (only!) 3000-6000 subjects.
- By contrast, a study in another elective orthopedic surgical population (e.g., hip arthroplasty) similar in design to STRIVE would take >10 years to complete and would require enrollment of 20,000 to 40,000 subjects.
- The AC thus focused on the question of whether (how) the results from one trial could generalize to other settings.
- I’ll also remind you of the recent Wellcome-sponsored report on antibacterial R&D opportunities which placed a S. aureus vaccine at the mid-point of difficulty:
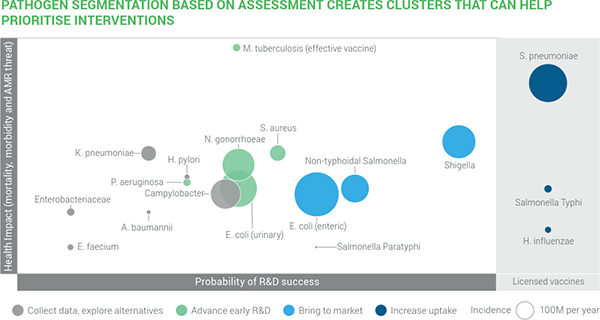
Deep sigh … we always knew vaccines were hard but something for S. aureus would be so valuable. There have been multiple prior failed efforts (Fowler & Proctor, CMI 2014) to create such a vaccine and Team Pfizer should be congratulated for giving this another serious try. I hope they will soon share their analyses on why this vaccine failed so that other work can be guided by their efforts.
“My strength lies solely in my tenacity.” Louis Pasteur
Onward! All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/
Upcoming meetings of interest to the AMR community:
- 15 Jan 2018 (London): BSAC’s Antimicrobial Chemotherapy Conference 2019: “An ABC for everyone involved in developing new antimicrobials.” Details here.
- 29 Jan 2018 (REVIVE webinar): “Clinical development for non-developers Part 3:
Antibacterial Drug Enhancer Combinations and Non-traditional Products.” Register here. - 4-5 Feb 2019 (London): Hamied Foundation UK-India Antimicrobial Resistance Meeting 2019. This is a 2-day meeting focused on building research links between the UK and India with the specific aim of jointly addressing the challenge of AMR. Register here.
- 14-15 Mar 2019 (Berlin): BEAM-, CARB-X-, and ND4BB-ENABLE-sponsored Berlin Conference on Novel Antimicrobials and AMR Diagnostics. Details here.
- 21-22 Mar 2019 (Birmingham, UK): BSAC Spring Conference.
- 26 Mar 2019 (London, UK): Sponsored by The Economist, a 1-day symposium entitled “Antimicrobial Resistance: Preventing an antibiotic apocalypse.” Register here.
- 13-16 Apr 2019 (Amsterdam): Annual ECCMID meeting.
- 24-26 Apr 2019 (Boston): Annual SHEA (Soc. for Hospital Epidemiology of America) Spring meeting
- 6-11 May 2019 (Ljubljana, Slovenia): 37th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID). Details here.
- 3-6 Jun 2019 (Philadelphia): Annual BIO meeting
- 20-24 June 2019 (San Francisco): Annual ASM Microbe meeting.
- [Mark your calendar now!] 3-6 Sep 2019 (Boston). Annual ASM-ESCMID Conference on Antibiotic Development. The Bootcamp series will continue on 3 Sep with the main meeting on 4-6 Sep. Mark your calendar now and check back here for details.
- 6-8 Sep 2019 (Bilbao, Spain): 5th ESCMID conference on Vaccines. Check back here for details.
- 2-6 Oct 2018 (Washington, DC): IDSA’s annual IDWeek meeting.
- 19-27 Oct 2019 (Annecy, France): International Course on Antibiotics and Resistance (ICARe) – A soup-to-nuts intensive residential training program on all things AMR, especially R&D for new antibiotics. See this link for details.