Dear All,
Based on a 2020 RFP, we’ve known that WHO was building a fungal priority pathogen list (PPL). Well, now the list has been released. Here’s what you need to understand the new PPL:
- Webpage where you can get the report
- My PowerPoint .pptx that summarizes all the PPLs to date
- My webpage that provides links to all the PPLs to date.
WHO’s motivation for this new PPL is based on its observation that “Fungal pathogens and infections are an increasing global public health concern” as supported by these excerpts from the opening background statement:
- “Cases of invasive fungal disease (IFD) are rising as the at-risk population continues to expand.
- …
- “People most at risk are those with underlying health problems or a weakened immune system, such as chronic lung disease, prior tuberculosis (TB), HIV, cancer, and diabetes mellitus.
- “Critically ill patients in an intensive care unit (ICU), patients undergoing invasive medical procedures and receiving broad-spectrum antibiotics, and those taking immune-suppressing medicines are also at risk
- …
- “The underrecognized and emerging global health threat of invasive fungal diseases is compounded by the rapid emergence of antifungal resistance and, in many settings, limited access to quality diagnostics and treatment”
- …
- “Despite posing a growing threat to human health, fungal infections receive very little attention and resources globally. This all makes it impossible to estimate the exact burden of fungal infections and consequently difficult to galvanize policy and programmatic action.”
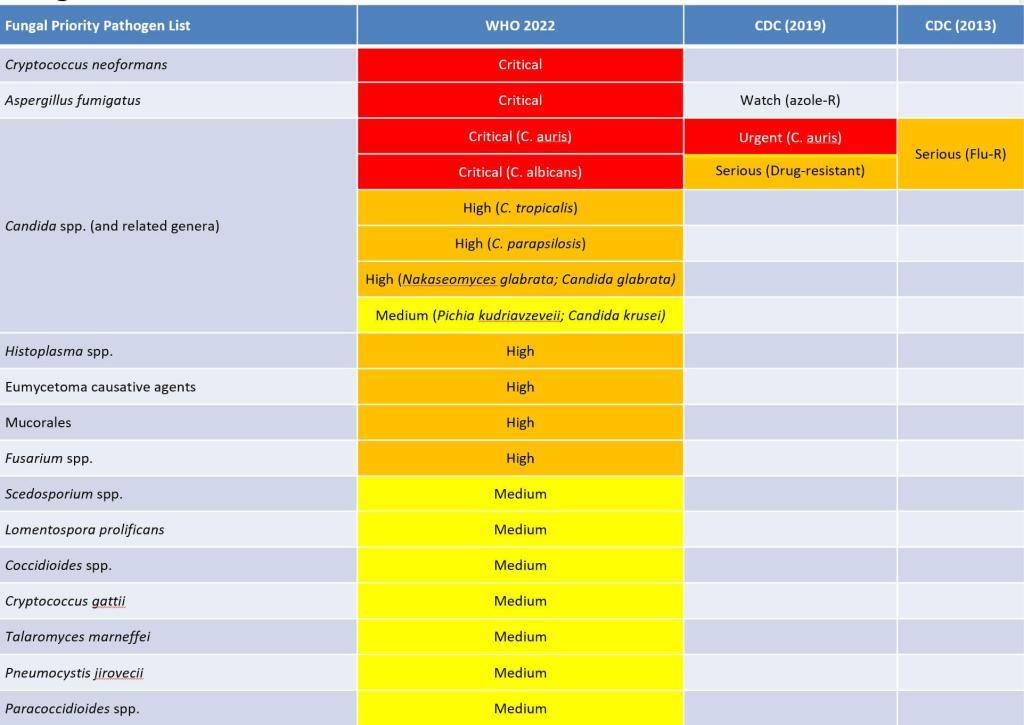
Well said! Divided into priority categories of Critical, High, and Medium based on weightings that considered factors such as deaths, annual incidence, resistance, and others based on input from multiple societies and expert groups (see the report), the WHO names 19 fungi (or groups of fungi) in this first fungus-focused PPL:
- Critical
- Cryptococcus neoformans
- Candida auris
- Aspergillus fumigatus
- Candida albicans
- High
- Nakaseomyces glabrata (Candida glabrata)
- Histoplasma spp.
- Eumycetoma causative agents
- Mucorales
- Fusarium spp.
- Candida tropicalis
- Candida parapsilosis
- Medium
- Scedosporium spp.
- Lomentospora prolificans
- Coccidioides spp.
- Pichia kudriavzeveii (Candida krusei)
- Cryptococcus gattii
- Talaromyces marneffei
- Pneumocystis jirovecii
- Paracoccidioides spp.
The authors of the report comment that (i) the ranking was heavily influenced by public health impact and antifungal resistance, (ii) there are major knowledge gaps on global burden, and (iii) the distribution/epidemiology of the fungi varied by region.
Well done, Team WHO! This new fungus-specific PPL is a thoughtful and comprehensive list (the fungus-by-fungus discussions are excellent) that will certainly encourage focused R&D. The only PPLs to previously mention fungi were from CDC and a visual summary of fungi named in a PPL is shown below. Note that Candida-related organisms are found in all three WHO categories but I’ve grouped them together for ease of reference:
To get an original of this graphic, see webpage and .pptx summary of PPLs to date.
It is excellent to have a PPL just for the fungi! Many thanks to WHO for this timely report!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- Current funding rounds from CARB-X are as described in this newsletter!
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- [NEW] 7 Nov 2022 (virtual, noon-6p GMT): Liverpool School of Tropical Medicine and NW England’s Infection Innovation Consortium (iiCON) are sponsoring an AMR Networking Event (noon-5p GMT; register here) that will be followed by a lecture by Professor Dame Sally Davies on “Antimicrobial resistance: where next?” (5.30-6p GMT; register here). Note there are TWO events here and you need to register separately for both.
- 17-20 Nov 2022 (Kuala Lumpur, Malaysia): The International Congress on Infectious Diseases will take place for the first time as a hybrid event. Go here for details.
- 18-24 Nov 2022 (Everywhere!): WHO’s World Antimicrobial Awareness Week is back and happening worldwide. This year, the theme of WAAW is “Preventing Antimicrobial Resistance Together.” Go here for the campaign details.
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.
- 3-7 Dec 2022 (Banff, Canada): Novel Approaches Against Emerging Antimicrobial Resistance by Keystone Symposia. Go here for details.
- [NEW] 14 Apr 2023 (Copenhagen, Denmark; 3-6.30p CEST): ECCMID and the Global Leaders Group on AMR will jointly sponsor a symposium entitled “Forging partnerships between science and policy in Antimicrobial Resistance (AMR).” Go here to register.
- [NEW] 15-18 Apr 2023 (Copenhagen, Denmark): 33rd ECCMID. Go here for details and to register.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.