Dear All (and with thanks to Kevin Outterson for being lead author on this newsletter),
(wonkish alert on this one … refresh your coffee and dig in!) Bonnifield and Towse of the Center for Global Development have released a blog post and a paper estimating the potential impact of the PASTEUR Act. Here’s what you need:
- Blog: “The World Needs New Antibiotics. A Proposed US Program to Develop Them Would Pay Off 28:1“
- Report: “An Ambitious USG Advanced Commitment for Subscription-Based Purchasing of Novel Antimicrobials and Its Expected Return on Investment”
- If you need a refresh on PASTEUR, see this 16 June 2021 newsletter or this 6-minute YouTube video. Briefly, it would purchase qualifying antibiotics for the US based on their medical value rather than their volume of use.
- 8 Dec 2022 update: There is a follow-up newsletter that gives values for Canada, Japan, UK, and EU
You’ll need to read the paper itself (set aside at least an hour for this … the thinking goes quite deep), but the essential point is that the authors have estimated PASTEUR’s value to the US (and the world) in terms both of lives saved and financial benefit:cost ratio should PASTEUR provide the global foundation for a Pull award to 18 new qualifying antibiotics over 30 years (that’s just 6 new drugs qualifying for PASTEUR per decade).
The results are astonishing: The US alone sees 383k lives saved and an ROI of 28:1. For the globe, lives saved total 9.9m with an ROI of 125:1 over 30 years! The methods used are discussed in more detail below our signatures, but the strength of this approach lies in its simplicity:
- The value of new qualifying antibiotics is built from years of life saved.
- The cost of the Pull award to the US government assumes an average award of $2.1b over 10 years to 6 qualifying new antibiotics per decade.
- The US contribution is assumed to be 46% of a global system of Pull awards (46% is the US share of G7+EU GDP).
- The math includes adjustments for inflation and discounting.
The top-line results are presented first for the domestic US economy (Table 1) and then for the global economy (Table 2):
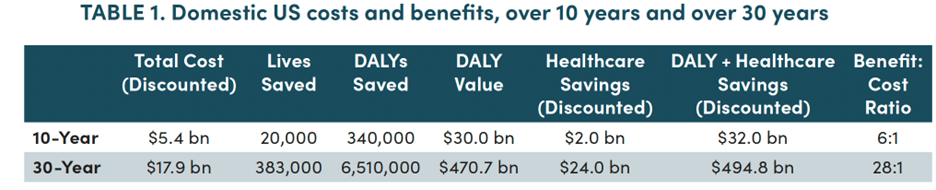
DALY = Disability-Adjusted Life Years; DALYs are assumed to convert 1:1 to QALYs (Quality-Adjusted Life Years); QALYs are assumed to have a value of $100k/year.
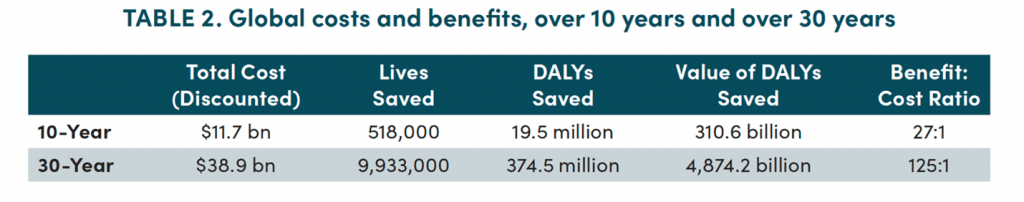
Yes … that’s an ROI of 28:1 for the US and 125:1 globally with 9.9 million lives saved over 30 years! Astounding!
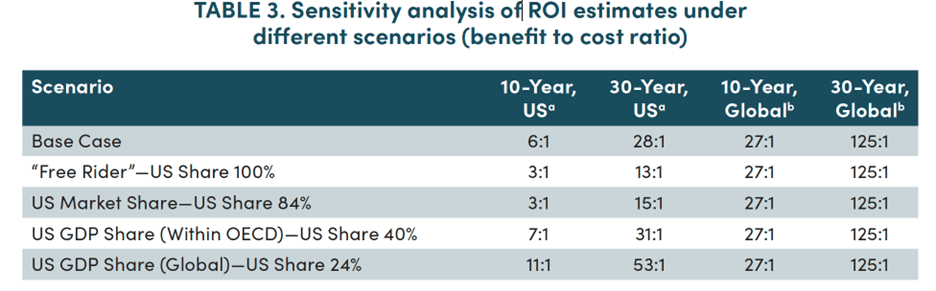
And importantly, the US ROI remains robust at 13:1 even if the the US shoulders the entire burden by providing 100% of the Pull (see the estimates labeled “Free Rider” in the 2nd line of Table 3, below; lives saved do not change):
These results are especially striking in that the model was built using a very simple approach that is different from prior pull incentive estimates. In particular, STEDI values were not used (for a refresher on STEDI, see this newsletter or this YouTube explainer).
Conservatively, the global health calculations also did not include additional benefits from saving health care costs as these data were not reliable on a global level. Productivity gains were also excluded, both domestically and globally, even though the World Bank found them to be substantial in their 2016 report..
Impressively, the core analysis demonstrated such high levels of cost-effectiveness from saving lives that there was no need to resort to secondary benefits.
This is as close to a slam dunk economic analysis as you will ever see. Their analysis is also congruent with the STEDI-based analyses used in the UK’s pilot showing that the high social value of antibiotics is evident even with very conservative estimates. Given their impressive social value, the funding proposed in the PASTEUR Act for compelling new antibiotics is truly a bargain.
—
The report also gives a clear account for why we have a market failure. These 8 key issues summarized on pp.4-6 of the report should be required reading for anyone trying to understand and fix the problem of AMR. Here’s their list along with our commentary:
- Initial sales volumes are low. Comment: Good stewardship makes this so; stewardship is great for public health but deadly for R&D companies.)
- Most social value is after patent expiry (Comment: This makes it difficult for a company to be appropriately rewarded during the patent period.)
- Clinical value is difficult to demonstrate (Comment: This surprises many when they first encounter the problem; for details, see previous newsletters on 19 Sep 2020 and 30 June 2020.)
- Traditional reimbursement approaches undervalue new antimicrobials (Comment: See discussions of the STEDI values (aka, the “fire extinguisher” values) of antibiotics in this newsletter and this YouTube video.)
- Common hospital payment mechanisms disincentivize use of novel (more expensive) antimicrobials (Comment: This is an unanticipated impact from the 1983 reforms that bundled hospital reimbursements in Medicare. The DISARM Act would address this issue.)
- Point of care diagnostic tests are not being used to reduce drug resistance (Comment: Like unhappy families in Tolstoy’s Anna Karenina, each market failure in AMR is broken in unique ways. For diagnostics, we await the release of the recent US National Academies of Science, Engineering, & Medicine report.)
- The science is difficult (Comment: This is undoubtedly true, but the experience of CARB-X suggests that the innovation teams around the world can overcome the scientific challenges if the economics were turned around.)
- Regulatory approval processes for new antimicrobials are time-consuming (Comment: While antibiotics might have been at some unique regulatory disadvantage in times past, this is no longer a top-tier reason for why antimicrobial innovation is harder than other drug classes. Regulators will continue to work, but we should celebrate progress to date.)
—
Wow … what a timely and impressive paper! The authors (Towse and Silverman) as well as their funders (Schmidt Futures and Founders Pledge) are indeed to be thanked for this valuable new contribution to the economic arguments in support of delinked Pull awards such as that proposed by the PASTEUR Act. Now let’s just get it passed! Have you written to your Members of Congressx?
All best wishes, Kevin & John
Kevin Outterson, JD, Professor of Law, Boston University & Executive Director, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of its funders) @koutterson
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Brief summary of the methodology
- The annual rate of AMR-attributable deaths was taken from the GRAM project report published earlier this year
- The report is in The Lancet: “Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis” (https://doi.org/10.1016/S0140-6736(21)02724-0).
- Note that there is now a second report from this project, also in The Lancet: “Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019” (https://doi.org/10.1016/S0140-6736(22)02185-7).
- Conservatively, they focused on attributable deaths from the 6 leading pathogens causing 79% of these deaths (E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa)
- They estimate lost DALYs (disability-adjusted life years) from these deaths using data from the GRAM report.
- DALYs are assumed to convert 1:1 to QALYs (quality-adjusted life years) and QALYS are valued at $100k/QALY.
- They assume that 18 new qualifying antibiotics are needed over 30 years for for these 6 pathogens (3 new drugs/pathogen) and that:
- The 18 new qualifying antibiotics appear steadily over that 30 years
- Each new drug is held completely in reserve for its first 4 years
- Each new drug reduces deaths/year by 5% from year 5 onward
- From year 6, each new drug loses effectiveness 2% year-on-year due to emergence of resistance
- Note that the focus on just the top 6 pathogens conservatively leaves a bit of room in the math for new qualifying drugs to cover more bugs
- They assume the Pull Award will provide (on average) a 10-year fully delinked award equal to the $4.2b central “best guess” required value that was estimated in Outterson 2021 “Estimating The Appropriate Size Of Global Pull Incentives For Antibacterial Medicines” (https://www.healthaffairs.org/doi/abs/10.1377/hlthaff.2021.00688).
- They adjust the numbers for inflation
- They use the same average value for all antibiotics that earn an award but they explicitly note that the actual reward size would need to be adjusted to reflect the merits of each qualifying antibiotic.
- Implicit in this discussion of antibiotic value is that antibiotics not providing interesting new coverage of the target pathogens would have zero value, again consistent with the notion that not all drugs would receive a Pull award.
- The US fair share of this amount is 46% based on proportion of GDP within the G7 + EU.
- This equates to $2.1b per drug from the US, a value that is roughly at the mid-point of the $750m to $3b range proposed as part of PASTEUR.
Current funding opportunities (most current list is here)
- FDA have announced five RFPs spanning antifungal animal models, usability of antimicrobial drug labeling, urine PK-PD, and interpretive breakpoints. Applications are due dates of 23 Jan 2023 — see this newsletter for more details.
- Current funding rounds from CARB-X are as described in this newsletter!
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.
- [NEW] 2 Dec 2022 (Harvard, Boston, 9a-6p with reception to follow): BAARN, the Boston Area Antimicrobial Resistance Network. A full day of networking and lectures, including a keynote by Gerry Wright. Go here for details and here to register.
- 3-7 Dec 2022 (Banff, Canada): Novel Approaches Against Emerging Antimicrobial Resistance by Keystone Symposia. Go here for details.
- [NEW] 1-2 Feb 2023 (virtual): Antimicrobial Chemotherapy Conference by GARDP and BSAC in collaboration with ReAct Africa and Africa CDC. Go here for details.
- 14 Apr 2023 (Copenhagen, Denmark; 3-6.30p CEST): ECCMID and the Global Leaders Group on AMR will jointly sponsor a symposium entitled “Forging partnerships between science and policy in Antimicrobial Resistance (AMR).” Go here to register.
- 15-18 Apr 2023 (Copenhagen, Denmark): 33rd ECCMID. Go here for details and to register.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.
- [NEW] 14-22 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
Dear All (and with thanks to Kevin Outterson for being lead author on this newsletter),
(wonkish alert on this one … refresh your coffee and dig in!) The Center for Global Development have released a blog post and a paper estimating the potential impact of the PASTEUR Act. Here’s what you need:
- Blog: “The World Needs New Antibiotics. A Proposed US Program to Develop Them Would Pay Off 28:1”
- Report: “An Ambitious USG Advanced Commitment for Subscription-Based Purchasing of Novel Antimicrobials and Its Expected Return on Investment”
8 Dec 2022: There is a follow-up newsletter that gives values for Canada, Japan, UK, and EU
- If you need a refresh on PASTEUR, see this 16 June 2021 newsletter or this 6-minute YouTube video. Briefly, it would purchase qualifying antibiotics for the US based on their medical value rather than their volume of use.
You’ll need to read the paper itself (set aside at least an hour for this … the thinking goes quite deep), but the essential point is that the authors have estimated PASTEUR’s value to the US (and the world) in terms both of lives saved and financial benefit:cost ratio should PASTEUR provide the global foundation for a Pull award to 18 new qualifying antibiotics over 30 years (that’s just 6 new drugs qualifying for PASTEUR per decade).
The results are astonishing: The US alone sees 383k lives saved and an ROI of 28:1. For the globe, lives saved total 9.9m with an ROI of 125:1 over 30 years! The methods used are discussed in more detail below our signatures, but the strength of this approach lies in its simplicity:
- The value of new qualifying antibiotics is built from years of life saved.
- The cost of the Pull award to the US government assumes an average award of $2.1b over 10 years to 6 qualifying new antibiotics per decade.
- The US contribution is assumed to be 46% of a global system of Pull awards (46% is the US share of G7+EU GDP).
- The math includes adjustments for inflation and discounting.
The top-line results are presented first for the domestic US economy (Table 1) and then for the global economy (Table 2):

DALY = Disability-Adjusted Life Years; DALYs are assumed to convert 1:1 to QALYs (Quality-Adjusted Life Years); QALYs are assumed to have a value of $100k/year.

Yes … that’s an ROI of 28:1 for the US and 125:1 globally with 9.9 million lives saved over 30 years! Astounding!
And importantly, the US ROI remains robust at 13:1 even if the the US shoulders the entire burden by providing 100% of the Pull (see the estimates labeled “Free Rider” in the 2nd line of Table 3, below; lives saved do not change):

These results are especially striking in that the model was built using a very simple approach that is different from prior pull incentive estimates. In particular, STEDI values were not used (for a refresher on STEDI, see this newsletter or this YouTube explainer).
Conservatively, the global health calculations also did not include additional benefits from saving health care costs as these data were not reliable on a global level. Productivity gains were also excluded, both domestically and globally, even though the World Bank found them to be substantial in their 2016 report..
Impressively, the core analysis demonstrated such high levels of cost-effectiveness from saving lives that there was no need to resort to secondary benefits.
This is as close to a slam dunk economic analysis as you will ever see. Their analysis is also congruent with the STEDI-based analyses used in the UK’s pilot showing that the high social value of antibiotics is evident even with very conservative estimates. Given their impressive social value, the funding proposed in the PASTEUR Act for compelling new antibiotics is truly a bargain.
—
The report also gives a clear account for why we have a market failure. These 8 key issues summarized on pp.4-6 of the report should be required reading for anyone trying to understand and fix the problem of AMR. Here’s their list along with our commentary:
- Initial sales volumes are low. Comment: Good stewardship makes this so; stewardship is great for public health but deadly for R&D companies.)
- Most social value is after patent expiry (Comment: This makes it difficult for a company to be appropriately rewarded during the patent period.)
- Clinical value is difficult to demonstrate (Comment: This surprises many when they first encounter the problem; for details, see previous newsletters on 19 Sep 2020 and 30 June 2020.)
- Traditional reimbursement approaches undervalue new antimicrobials (Comment: See discussions of the STEDI values (aka, the “fire extinguisher” values) of antibiotics in this newsletter and this YouTube video.)
- Common hospital payment mechanisms disincentivize use of novel (more expensive) antimicrobials (Comment: This is an unanticipated impact from the 1983 reforms that bundled hospital reimbursements in Medicare. The DISARM Act would address this issue.)
- Point of care diagnostic tests are not being used to reduce drug resistance (Comment: Like unhappy families in Tolstoy’s Anna Karenina, each market failure in AMR is broken in unique ways. For diagnostics, we await the release of the recent US National Academies of Science, Engineering, & Medicine report.)
- The science is difficult (Comment: This is undoubtedly true, but the experience of CARB-X suggests that the innovation teams around the world can overcome the scientific challenges if the economics were turned around.)
- Regulatory approval processes for new antimicrobials are time-consuming (Comment: While antibiotics might have been at some unique regulatory disadvantage in times past, this is no longer a top-tier reason for why antimicrobial innovation is harder than other drug classes. Regulators will continue to work, but we should celebrate progress to date.)
—
Wow … what a timely and impressive paper! The authors (Towse and Silverman) as well as their funders (Schmidt Futures and Founders Pledge) are indeed to be thanked for this valuable new contribution to the economic arguments in support of delinked Pull awards such as that proposed by the PASTEUR Act. Now let’s just get it passed! Have you written to your Members of Congressx?
All best wishes, Kevin & John
Kevin Outterson, JD, Professor of Law, Boston University & Executive Director, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of its funders) @koutterson
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Brief summary of the methodology
- The annual rate of AMR-attributable deaths was taken from the GRAM project report published earlier this year
- The report is in The Lancet: “Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis” (https://doi.org/10.1016/S0140-6736(21)02724-0).
- Note that there is now a second report from this project, also in The Lancet: “Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019” (https://doi.org/10.1016/S0140-6736(22)02185-7).
- Conservatively, they focused on attributable deaths from the 6 leading pathogens causing 79% of these deaths (E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa)
- They estimate lost DALYs (disability-adjusted life years) from these deaths using data from the GRAM report.
- DALYs are assumed to convert 1:1 to QALYs (quality-adjusted life years) and QALYS are valued at $100k/QALY.
- They assume that 18 new qualifying antibiotics are needed over 30 years for for these 6 pathogens (3 new drugs/pathogen) and that:
- The 18 new qualifying antibiotics appear steadily over that 30 years
- Each new drug is held completely in reserve for its first 4 years
- Each new drug reduces deaths/year by 5% from year 5 onward
- From year 6, each new drug loses effectiveness 2% year-on-year due to emergence of resistance
- Note that the focus on just the top 6 pathogens conservatively leaves a bit of room in the math for new qualifying drugs to cover more bugs
- They assume the Pull Award will provide (on average) a 10-year fully delinked award equal to the $4.2b central “best guess” required value that was estimated in Outterson 2021 “Estimating The Appropriate Size Of Global Pull Incentives For Antibacterial Medicines” (https://www.healthaffairs.org/doi/abs/10.1377/hlthaff.2021.00688).
- They adjust the numbers for inflation
- They use the same average value for all antibiotics that earn an award but they explicitly note that the actual reward size would need to be adjusted to reflect the merits of each qualifying antibiotic.
- Implicit in this discussion of antibiotic value is that antibiotics not providing interesting new coverage of the target pathogens would have zero value, again consistent with the notion that not all drugs would receive a Pull award.
- The US fair share of this amount is 46% based on proportion of GDP within the G7 + EU.
- This equates to $2.1b per drug from the US, a value that is roughly at the mid-point of the $750m to $3b range proposed as part of PASTEUR.
Current funding opportunities (most current list is here)
- FDA have announced five RFPs spanning antifungal animal models, usability of antimicrobial drug labeling, urine PK-PD, and interpretive breakpoints. Applications are due dates of 23 Jan 2023 — see this newsletter for more details.
- Current funding rounds from CARB-X are as described in this newsletter!
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.
- [NEW] 2 Dec 2022 (Harvard, Boston, 9a-6p with reception to follow): BAARN, the Boston Area Antimicrobial Resistance Network. A full day of networking and lectures, including a keynote by Gerry Wright. Go here for details and here to register.
- 3-7 Dec 2022 (Banff, Canada): Novel Approaches Against Emerging Antimicrobial Resistance by Keystone Symposia. Go here for details.
- [NEW] 1-2 Feb 2023 (virtual): Antimicrobial Chemotherapy Conference by GARDP and BSAC in collaboration with ReAct Africa and Africa CDC. Go here for details.
- 14 Apr 2023 (Copenhagen, Denmark; 3-6.30p CEST): ECCMID and the Global Leaders Group on AMR will jointly sponsor a symposium entitled “Forging partnerships between science and policy in Antimicrobial Resistance (AMR).” Go here to register.
- 15-18 Apr 2023 (Copenhagen, Denmark): 33rd ECCMID. Go here for details and to register.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.
- [NEW] 14-22 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.