Dear All,
Greetings from ECCMID 2023 in Copenhagen! The days are busy, but last night we had a joint session between ECCMID and the Global Leaders Group on Antimicrobial Resistance (GLG) entitled “Forging partnerships between science and policy: A high-level Antimicrobial Resistance (AMR) event” had an excellent scientific program focused on prevention and use of diagnostics, but I would like to focus this newsletter on the energizing closing comments on high-level political action by Haileyesus Getahun, (Haile) MD, MPH, PhD who is Director of the Quadripartite Joint Secretariat on AMR for WHO.
To tell the story, let’s use 4 slides from his talk:
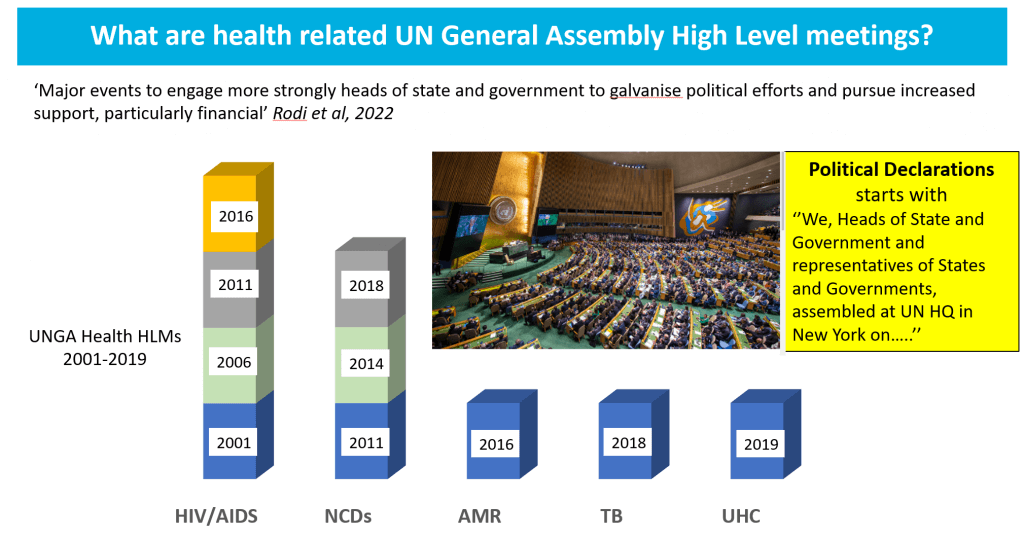
Since 2001, there have been 10 high-level health-related political events. In 2016, UNGA (the UN General Assembly) focused on AMR for the first time!
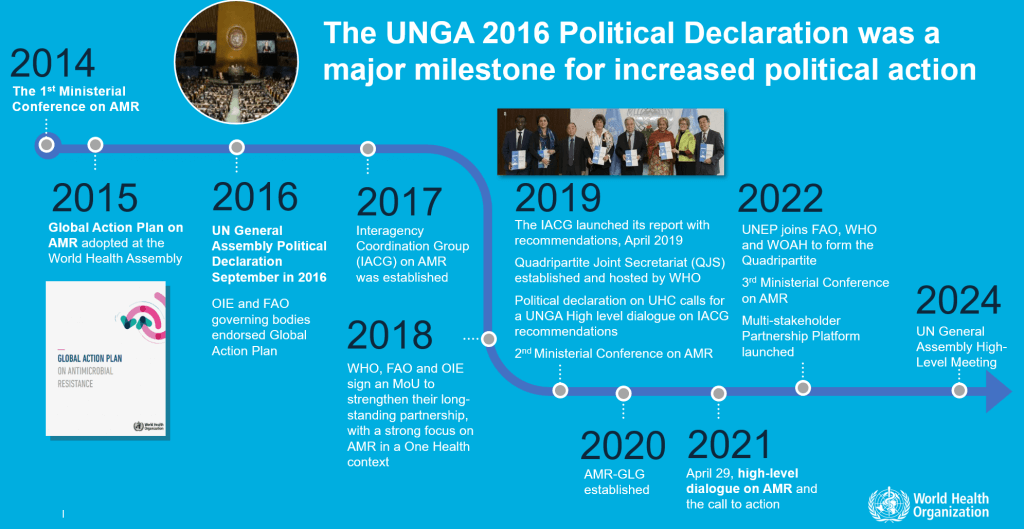
UNGA 2016 has put momentum into multiple subsequent political events: Creation of the IACG, of the GLG-AMR itself, and now the upcoming UNGA 2024 event.
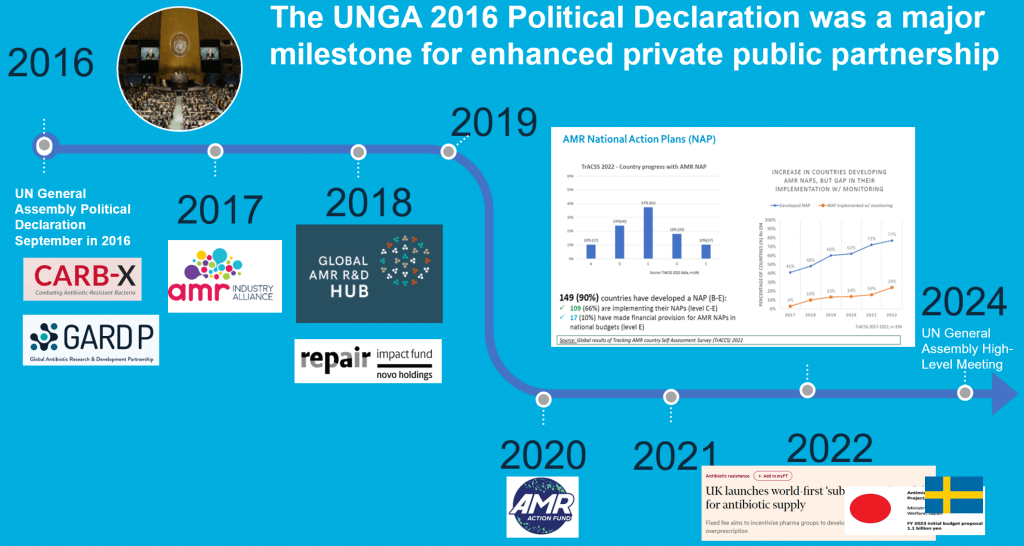
UNGA 2016 also helped catalyze a series of public-private partnerships: CARB-X, GARDP, the AMR Industry Alliance, the Global AMR R&D Hub, the Novo REPAIR Impact Fund, the AMR Action Fund, and (drum roll, please) the UK NHS subscription pilot creating the world’s first Pull incentive at a scale the would drive innovation. And there’s more not shown here … we know of action underway in Japan, Canada, and the EU/EC.
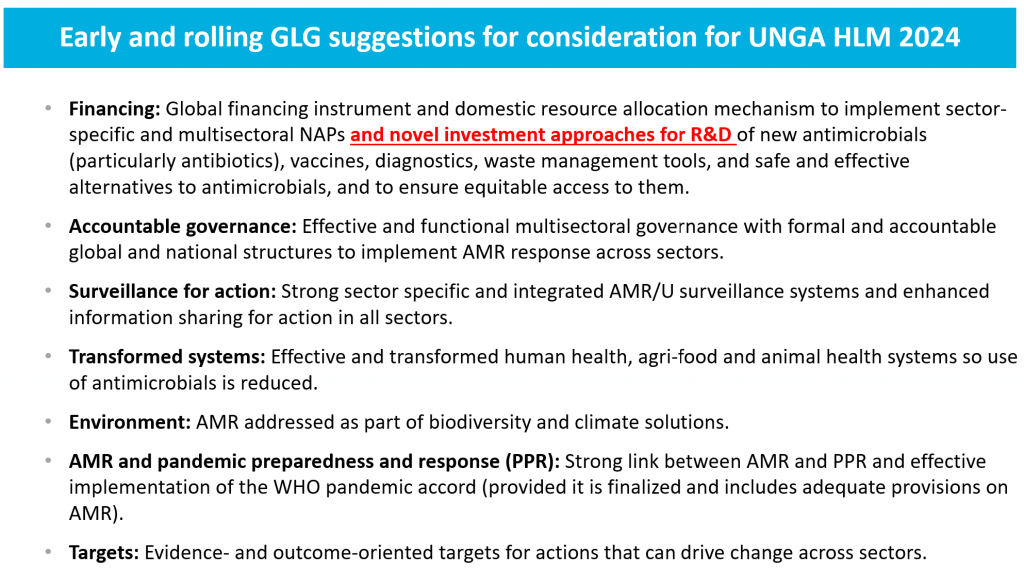
And then we have the emerging AMR agenda for UNGA 2024 where point #1 is financing. Here we certainly hope to see more action on Pull — I am in particular very hopeful that we’ll see PASTEUR be reintroduced into Congress and pass in the United States. Its global impact would be huge: over a 30-year period, it would save a total of 9.9m lives and have an ROI of 125:1!
Wow! We’ve come a looooooooooooong way since the IDSA’s 2004 call to action (“Bad Bugs, No Drugs: As Antibiotic R&D Stagnates, a Public Health Crisis Brews”), ESKAPE (the first priority pathogen list), and the ground-breaking 2009 conference (“Innovative incentives for Effective Antibacterials”) hosted by the Swedish EU Presidency. Twenty years of effort … amazing!
What does this mean for you? Well, all of us should make our voices heard! Action from societies (position papers!), companies (endorse those position papers), and individuals (endorse those position papers; write your local political leadership).
With thanks to all of you who are supporting this grand effort … onward!, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- [UPDATED – New application round] CARB-X again has an open-round for funding applications with a deadline of 1 May 2023. Applications are sought for any of 3 themes (oral products, vaccines for neonatal sepsis, gonorrhea products) as described in this newsletter!
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 15-18 Apr 2023 (Copenhagen, Denmark): 33rd ECCMID. Go here for details and to register.
- 26 Apr 2023 (virtual, noon-1.30p CET): Webinar entitled “WHO Human health AMR research agenda” from WHO’s series entitled “WHO Global Webinar Series to Support Implementation of National Action Plans on Antimicrobial Resistance (AMR).” Go here to register.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.
- 3-5 Jul 2023 (Tours, France): 9th Symposium on Antimicrobial Resistance in Animals and the Environment (ARAE). Sponsored by INRAE (French National Research Institute for Agriculture, Food, and Environment, itself a merger of merger of INRA, the French National Institute for Agricultural Research, and IRSTEA, the French National Research Institute of Science and Technology for the Environment and Agriculture), this conference has been running since 2005. Go here for details.
- [NOW OPEN FOR APPLICATIONS!] 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.