Dear All (and with thanks to Kevin for co-authoring):
Yesterday saw the release of a major new report on Pull incentives from Canada! The report’s cover art eloquently summarizes its key message:
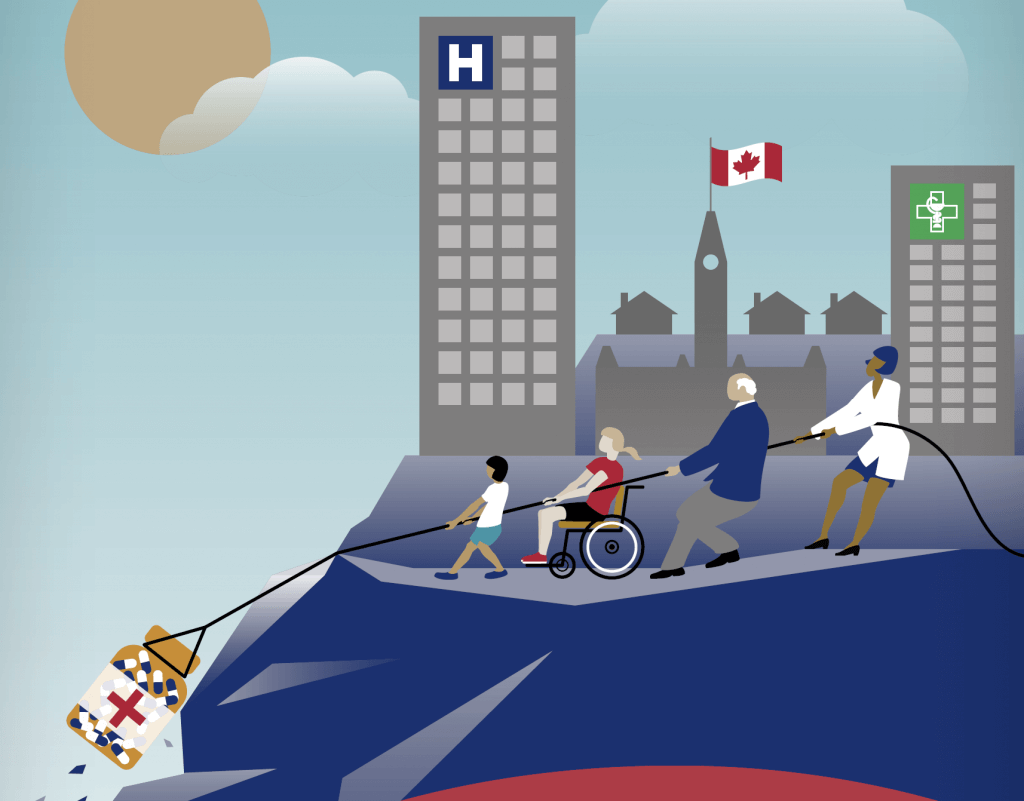
Well said … and proves that a picture really is worth 1,000 words! Here are the links you’ll need:
- The report’s webpage at the Canadian Council of Academies (CCA)
- The report itself: “Overcoming Resistance”
- A one-pager on the report
- A short slide deck presented by Andrew Morris, the Report Chair
- And a disclaimer: Kevin and I were part of the expert panel who support the writing of the report
- Also useful to contemplate in parallel
- The 2021 G7 Shared Principles for the Valuation of Antimicrobial Therapeutics
- “We will develop a set of shared valuation principles, based on public health needs and taking into account the WHO priority pathogen list, “
- The April 2023 paper from the Global AMR R&D Hub entitled “A shared dialogue on Pull incentives”
- “For any pull incentive, it is critical that a global perspective is pursued” and
- “Although countries have their own priority needs & burden, alignment across countries will help support predictability. “
- The 2021 G7 Shared Principles for the Valuation of Antimicrobial Therapeutics
The excellent new report from the CCA is a great example of the theme “Think globally, act locally” that is emphasized by the reports from the G7 (2021) and the AMR Hub (2023).
In brief, the report was grounded in the recognition that (antibacterial) AMR threatens the health of all Canadians and the fact that antibiotic access in Canada lagged that of other advanced economies (Outterson 2021). Based on this, the Public Health Agency of Canada (PHAC) worked with Health Canada to ask the CCA to provide an evidence-based analysis of which “economic pull incentives have the greatest potential for success in encouraging the market entry and sustained market availability of high-value antimicrobials for use in humans in Canada.”
The core conclusion of the report is that a Subscription-style Pull Incentive (SPI) similar to that of the UK but adapted to the Canadian context (health care is delivered by Provinces and Territories, not a national single payer) could be used to support the creation of innovative new agents that would benefit Canadians and the world. Making it concrete, an average of CAN$14.5 to CAN$18 million per year per agent for 10 years would be a fair share for Canada to contribute for novel antimicrobials showing real medical value. We agree – this amount reflects Canada’s proportionate share of GDP within the G7+EU27.
The report also emphasizes the need for global collaboration highlighted by the companion papers cited above when it says: “Canada has the opportunity to work with a group of other high income countries to contribute its fair share to an adequate global pull incentives.” As emphasized by the companion papers, we need aligned targets and approaches to valuation. Here again the UK’s leadership with developing the STEDI principles and the WHO’s work to create globally relevant Priority Pathogen Lists provide an invaluable starting points.
Finally, the report provides an interesting insight into the way that success for Canada as a whole would require rely on collaboration among the federal, provincial, and territorial governments that manage Canada’s healthcare systems.
MANY thanks to Team Canada for jumping into the AMR fray first with their 2019 report When Antibiotics Fail and then with this superb report. With all of the G7 now both supporting Push and engaged in discussions of Pull, it feels like we are closer than ever to a durable solution — this report will provide valuable additional momentum during the High-Level Meeting (HLM) on AMR at UNGA 2024!
All best wishes, John & Kevin
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Kevin Outterson, JD, Professor of Law, Boston University & Executive Director, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of its funders) @koutterson
Current funding opportunities (most current list is here)
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. Applications are accepted on a rolling basis; go here for all the details.
- The ENABLE-2 consortium has announced a call to support hit-to-lead compound development by researchers at publicly-funded European universities. The call is focused on molecules with the potential to be direct-acting therapies for one or more of the following priority pathogens: ESBL-producing/carbapenem-resistant Enterobacteriaceae (E. coli, K. pneumoniae), P. aeruginosa, A. baumannii, methicillin-resistant S. aureus, or vancomycin-resistant E. faecium. The Call is open continuously, applications are reviewed at intervals, and funding is non-dilutive. Expressions of interest received before 30 Sep 2023 would be considered in November 2023. Applications received after this date will be evaluated in the spring of 2024 (date to be decided). Go to https://www.ilk.uu.se/enable2/apply/ for further details.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- [Last call!] 8 Sep 2023 (deadline): Not a meeting but rather an opportunity to sign a letter calling for the passage of the PASTEUR Act. Organized by The Valley Fever Institute at Kern County Medical Center, the letter to California’s Members of Congress calls for passage of PASTEUR as a support for new therapies for Valley Fever (coccidioidomycosis). Go here to sign the letter.
- 18 Sep 2023 (virtual, 3-4p CET): Jointly hosted by the US CDC and the WHO, a webinar entitled “Fungal Diseases – Global Challenges and Opportunities” will kick-start CDC’s Fungal Disease Awareness Week (FDAW). The goal of the webinar is to provide an in-depth perspective on fungal diseases and discuss challenges related to antifungal resistance. Go here to register; the full program for the week can be seen at the FDAW webpage.
- 19-22 Sep 2023 (Boston, USA): ASM-ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. This is an excellent development focused meeting … highly recommended! Go here for details and to register.
- 21 Sep 2023 (New York City, USA, in person 6.30-8.00p ET): In partnership with the governments of the UK and Nigeria, the Center for Global Development (CGD) will launch its final report from a CGD working group studying possible purchasing systems that will improve access, stewardship, and innovation for antimicrobials in LMICs at an event entitled “A Path Towards Affordable and Effective Antimicrobials: Six Recommendations to Improve Antimicrobial Innovation, Access, and Stewardship,” Go here for more details and to register: the event will be held at the Sixth Floor Loft (873 Broadway, Sixth Floor, New York, NY 10003). The discussion will focus on the political and operational steps needed to ensure access to and stewardship of affordable and effective antimicrobials — and you can readily see how this is is step towards building consensus around priorities for the discussion of next year’s high-level meeting (HLM) at UNGA 2024.
- [NEW] 4 Oct 2023 (Museum of Science, Boston, MA; in person, 11.30a-2.30p ET): Co-sponsored by the Boston’s Museum of Science and the Rijksmuseum Boerhaave (Netherlands), you can attend a luncheon and panel discussion entitled “Celebrating 300 Years of Innovation and Beyond: How van Leeuwenhoek’s Discovery of the Microworld Sparked a Medical Revolution, with a Focus on Antimicrobial Resistance.” Go here for details and to register. I knew about Boston’s MOS but did not know about the exceptional collection of the Rijksmuseum Boerhaave which seeks to “… show what science is all about: curiosity, guts, creativity and perseverance.” Fascinating!
- 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- 11-15 Oct 2023 (Boston, USA): IDWeek 2023, the annual meeting of the Infectious Diseases Society of America. Go here for details and to register.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.
- 6-7 Feb 2024 (online): Antimicrobial Chemotherapy Conference. This is an annual, free of charge conference that is co-organized by GARDP and the British Society for Antimicrobial Chemotherapy (BSAC). Details to follow — for now, just mark your calendar.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.