Dear All (wonkish alert … but definitely worth the effort! Refill your coffee!),
Ramanan Laxminarayan and about 40 colleagues from around the world have just published in the Lancet a GLORIOUS collection of papers on the theme of “Sustainable Access to Effective Antibiotics.”
There are six papers in total for you to digest: An Executive Summary, four papers with analysis and data, and then a commentary. Here are the links you need along with very brief comments (and see below my signature for a more extended tour of the papers):
- The overview webpage for the series: “Sustainable Access to Effective Antibiotics.”
- Okeke et al., Executive Summary: Lancet Series on Sustainable Access to Effective Antibiotics
- Okeke et al., Sustainable Access to Antibiotics 1: The scope of the antimicrobial resistance challenge
- Key message: (bacterial) AMR is a major barrier to achieving the UN SDGs (Sustainable Development Goals)
- Lewnard et al., Sustainable Access to Antibiotics 2: Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: an evidence review and modelling analysis
- Key messages:
- WASH, vaccines, and better IPC can avert some (but not all) of AMR-related burden
- Significantly, a 10% reduction in mortality is within reach with these existing tools!
- Key messages:
- Laxminarayan et al., Sustainable Access to Antibiotics 3: Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance
- Key messages:
- To go further, progress on antibiotics, vaccines, and diagnostics (new and old) aligned with access is needed
- And for this to work, we need Push & Pull … remember that there is no access for anyone to innovative therapies unless we create and sustain them!
- Key messages:
- Mendelson et al., Sustainable Access to Antibiotics 4: Ensuring progress on sustainable access to effective antibiotics at the 2024 UN General Assembly: a target-based approach
- Key message: These actionable “Lancet targets” would move us forward:
- 10% global reduction in AMR-related deaths by 2030
- 20% global reduction in inappropriate human antimicrobial use
- 30% global reduction in inappropriate animal antimicrobial use
- And this needs oversight: We need an Independent Panel on Evidence
- Key message: These actionable “Lancet targets” would move us forward:
- Shamas et al., Commentary: Antimicrobial resistance survivors: Calling the world to action by the WHO Task Force of AMR Survivors
- Key message: AMR can affect anyone, regardless of wealth, race, or region across the globe.
- Also, watch for their upcoming campaign called “Antimicrobial resistance (AMR) is invisible. I am not” … what a great message!
- And if you’d prefer to listen, Ramanan and I were able to record a 30-minute Fireside chat in which we take a video tour of the papers:
- And if you need more on UNGA 2024 and the HLM on AMR (High-Level Meeting on AMR)
- This 3 May 2024 newsletter is a great place to start
- I have been compiling key reports on this webpage
GREAT stuff! The “10-20-30 target by 2030” provides a clear and actionable message for policymakers. Simplicity is very much a virtue here! We can make progress!
And to reiterate, heart of the message is that we can make progress with existing tools to a point … but beyond that we need access to new and old therapies, vaccines and diagnostics.
Whew! Don’t just take my word for it, read the papers, watch the video, or both and then share this newsletter with anyone you think could help speak out about the importance of AMR awareness before UNGA meets in September!! You can also talk to or write to your government representatives and start planning for WHO AMR Awareness week 2024 (18-24 November 2024)! Even a social media post tagging your representative sends a notification to their office — no effort on the path is wasted!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
My summary of key points from the papers
- Paper #1: Okeke et al.: Sustainable Access to Antibiotics 1: The scope of the antimicrobial resistance challenge
- (Bacterial) AMR is now a major barrier to attainment of SDGs
- TB, HIV, and malaria are big, but bacterial AMR as a whole is bigger and (more) global.
- 178 countries have NAPs (National Action Plans) on AMR but only 25% are effectively implementing them! Oh dear!
- The previous 2016 Lancet series on AMR noted that more deaths were due to poor access to antibiotics rather than resistance.
- As discussed in the 29 April 2024 newsletter entitled “R&D Implications: Global Burden of Disease Is 28% Infectious!”, the IHME data on Global Burden of Disease found that the DALYs (Disability-Adjusted Life Years) destroyed in 2019 by infection were:
- 171m DALYs: TB + HIV + malaria
- 204m DALYs: The top 7 bacteria (Streptococcus spp., S. aureus, E. coli, K. pneunoniae, P. aeruginosa, A. baumannii, Enterococcus spp.)
- We need enhanced global surveillance built on national surveillance systems and better diagnostics. The more data we have, the better!
- (Bacterial) AMR is now a major barrier to attainment of SDGs
- Paper #2: Lewnard et al., Sustainable Access to Antibiotics 2: Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: an evidence review and modelling analysis
- About 1/5 (~18%, to be precise) of AMR-related burden can be averted by using WASH (Water, Sanitation and Hygiene), vaccines, and better IPC (Infection Prevention and Control)
- Hand hygiene is the single most powerful tool at our disposal!
- We can thank COVID-19 for creating public awareness of how to handwash properly but we need to keep going!
- We do need to be realistic however: Real-world WASH interventions have not been consistent in reducing the burden.
- Vaccinations are always good! As is good antibiotic stewardship! But there is only so much all these things can do before they hit their limits.
- Success here would get us close to the 2030 SDG targets…but no further…these tools have limits.
- “Total preventable burden across all three classes of interventions represents approximately 18% of AMR-associated deaths occurring in LMICs annually.” WOW!
- About 1/5 (~18%, to be precise) of AMR-related burden can be averted by using WASH (Water, Sanitation and Hygiene), vaccines, and better IPC (Infection Prevention and Control)
- Paper #3: Laxminarayan et al., Sustainable Access to Antibiotics 3: Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance
- To go beyond those targets, progress on antibiotics, vaccines, and diagnostics (new and old) aligned with access is needed
- New tools are needed alongside access to old and new:
- 2016 Lancet paper: lack of access caused more deaths than resistance.
- R&D of new antibiotics is no longer economically viable
- Push! Public-private partnerships such as CARB-X and GARDP play a crucial role to support the most promising R&D projects while obtaining guarantees that the new antibiotics will be marketed with appropriate stewardship and access.
- Pull! We need to pass the PASTEUR Act in the US and implement some form of Pull in Europe (e.g., the European Commission’s 2-step TEV to delinked model approach [2 May 2023 newsletter] or the pan-EU/EEA flexible revenue guarantee led by HERA [4 Dec 2023 newsletter], or some combination thereof).
- We also need to be sure we invest in priority areas. See the figure just below showing the distribution of R&D spend. I don’t want to spend less on HIV, TB, or Malaria … but we definitely need more on AMR!
- From the paper: “The architecture for deploying drugs and diagnostics for the big 3 diseases—HIV, malaria, and tuberculosis—has been well developed with entities such as the Global Fund to Fight AIDS, Tuberculosis, and Malaria and Unitaid. Similar procurement and market-shaping support are absent for antibiotics, even though non-tuberculosis bacterial infections kill 7.7 million people each year—far more than HIV, malaria, and tuberculosis combined.”
- See also the 29 Apr 2024 newsletter for a discussion of the balance of R&D spend vs. burden of disease from specific pathogens.
- Finally, diagnostics face distinct technical, economic, and behavioral challenges that require distinct solutions.
- New tools are needed alongside access to old and new:
- To go beyond those targets, progress on antibiotics, vaccines, and diagnostics (new and old) aligned with access is needed
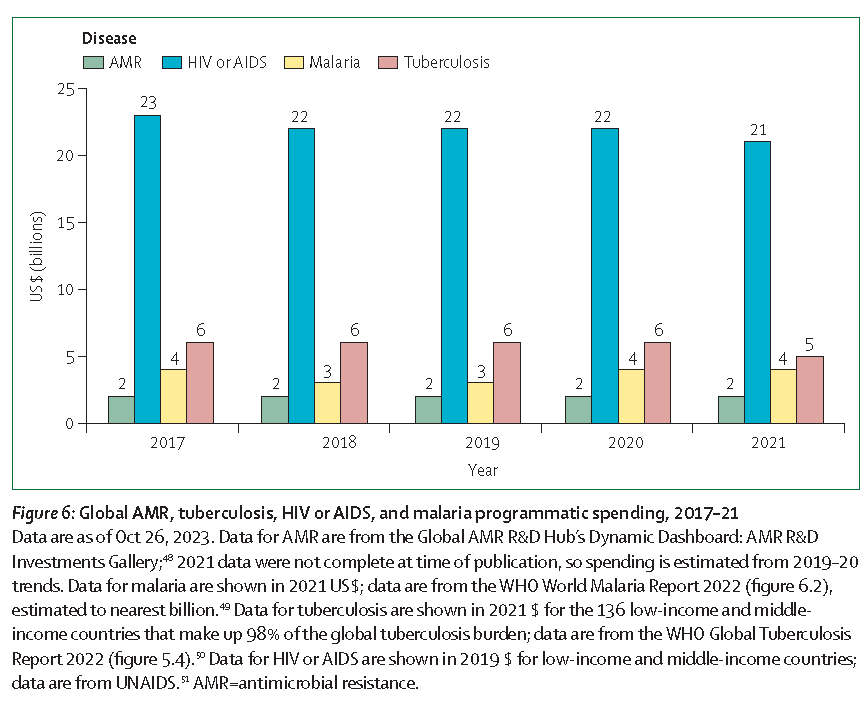
The figure above is from paper #4.
- Paper #4: Mendelson et al., Sustainable Access to Antibiotics 4: Ensuring progress on sustainable access to effective antibiotics at the 2024 UN General Assembly: a target-based approach
- 10-20-30 targets proposed for UNGA 2024:
- 10% global reduction in AMR-related deaths
- 20% global reduction in inappropriate human antimicrobial use
- Sub-goal: Universal accessibility of essential Access antibiotics in primary care settings (see the WHO AWaRe classification)
Sub-goal: Achieve risk-adjusted country level agreed targets for use of Total and Access/Watch/Reserve antibiotics
- Sub-goal: Universal accessibility of essential Access antibiotics in primary care settings (see the WHO AWaRe classification)
- 30% global reduction in inappropriate animal antimicrobial use
- Overseen by an Independent Panel on Evidence
- See also the 19 Apr 2024 newsletter about the GLG AMR’s similar call for such a panel
- 10-20-30 targets proposed for UNGA 2024:
- CARB-X has open calls that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- [REGISTRATION IS NOW OPEN] 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to register!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
Upcoming meetings of interest to the AMR community:
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 28-29 May 2024 (in person, Uppsala, Sweden): Uppsala Antibiotic Days, a broad-ranging 2-day program hosted by the Uppsala Antibiotic Center. Go here for details and to register.
- 30-31 May 2024 (face-to-face in Rockville, Maryland as well as online, 8.30-5.30p ET on 30 May, 9-2.40p on 31 May): NIAID-sponsored workshop entitled “Towards realizing the promise of adjunctive immune therapy for invasive fungal infections”. The agenda covers host immunity to invasive fungal infections, immune modulators in the context of fungal infections; and strategies for testing immune modulators as adjunctive therapy. Go here for more details and to register.
- [NEW] 31 May 2024 (virtual, 13:00 – 14:15 CEST), a “The Strategic Round Table on AMR” hosted by the WHO’s AMR team that will “convene global AMR leaders to discuss how Member States can best seize the opportunities of 2024 to accelerate and broaden the response to AMR beyond the health sector.” Go here to listen to the webcast.
- 9-13 June 2024 (in person, Ascona, Switzerland): “New Approaches to Combat Antibiotic-Resistant Bacteria, 2nd Edition” is a Sunday-Thursday residential workshop focused on the deep biology of AMR. Sponsored by NCCR AntiResist (a Swiss National Science Foundation consortium), the scientific program has the feel of a Gordon Conference. Space is limited, so you are encouraged to apply promptly — go here for details.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- [NEW] 27 June 2024 (virtual, 5p-630p CEST): GARDP REVIVE Webinar “Progressing an antibacterial drug discovery project – an SME perspective”. Click here for details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.