17 Sep 2024 update: This is now the second of a 3-part newsletter series! See also part 1 (“#AMRSOS! GRAM report: ‘at least 1.27m deaths/year directly attributable to AMR’“) and part 3 (17 Sep 2024: “#AMRSOS! AMR could worsen, killing 39m during 2025-2050”).
Dear All (with thanks to Erin Duffy for co-authoring and with a wonkish alert! Refresh your coffee!),
In a fascinating follow-up to their 2022 paper on the global burden of antimicrobial resistance (20 Jan 2022 newsletter entitled “#AMRSOS! GRAM Report: ‘At Least 1.27m Deaths/Year Directly Attributable To AMR'”), the team at IHME (Institute for Health Metrics and Evaluation) have published an analysis of the global burden of disease due to infection (Naghavi et al., Lancet ID 2024).
The punchline is that 28% of the global burden of disease (all causes) is due to infections and (drum roll) half of that (14% of the global burden) is due to bacterial infections. But to understand how we get to these numbers, a bit of background is needed … so settle in for a a short tour:
- Starting in 1991, the GBD (Global Burden of Disease) project began to systematically quantify and summarize all causes of disease on a global basis.
- For an overview of the project, see Murray CJL, “The Global Burden of Disease Study at 30 years,” Nature Medicine 2022.
- A core measure used by the project is that of DALYs (disability-adjusted life-years) that are lost due to disease.
- DALYs encompass both mortality and morbidity.
- In technical terms, DALY is the sum of YLL (Years of Life Lost) and YLD (Years Lived with Disability)
- In 2019, the GBD project estimates that 2,540 million (2.54 billion) DALYs were lost due to illnesses of all causes (disease or injury)
- (moving now to the new paper), the GBD project has analyzed infectious causes of DALYs for 85 pathogens (or groups of pathogens). In 2019 (pre-COVID), we have these stunning totals:
- Infections caused 704m (28%) of the global burden of 2,540m DALYs.
- Of those, half (14%, 350m DALYs) were due to bacteria (see footnote below signatures).
- Tuberculosis (65.1m), malaria (53.6m), and HIV-AIDS (52.1m) were of course also individually big, totaling 170.8m DALYs.
- Of the bacterial causes, just 7 bacteria collectively destroyed 203.8m DALYs (8% of the total global burden from all causes) and were:
- Streptococcus spp. (56.0m, combining all the Streptococcus categories in the report)
- Staphylococcus aureus (34.5m)
- Escherichia coli (31.3m, combining all the E. coli categories in the report)
- Klebsiella pneumoniae (31.1m)
- Pseudomonas aeruginosa (18.3m)
- Acinetobacter baumannii (16.7m)
- Enterococcus spp. (15.9m, combining all the Enterococcus categories)
- Post-newsletter addendum: In the original newsletter, we failed to combine all the Streptococcus categories, we inadvertently included E. histolytica as a bacterium, and we failed to recognize the grouping of the Enterococcus spp. Thus, the overall tally for the top 7 now updates the DALYs for Streptococcus from 38.1 to 56.0, adds Enterococcus (15.9m), and removes Helicobacter (16.4). With this, the total DALYs destroyed by the top 7 bacteria is 203.8m.
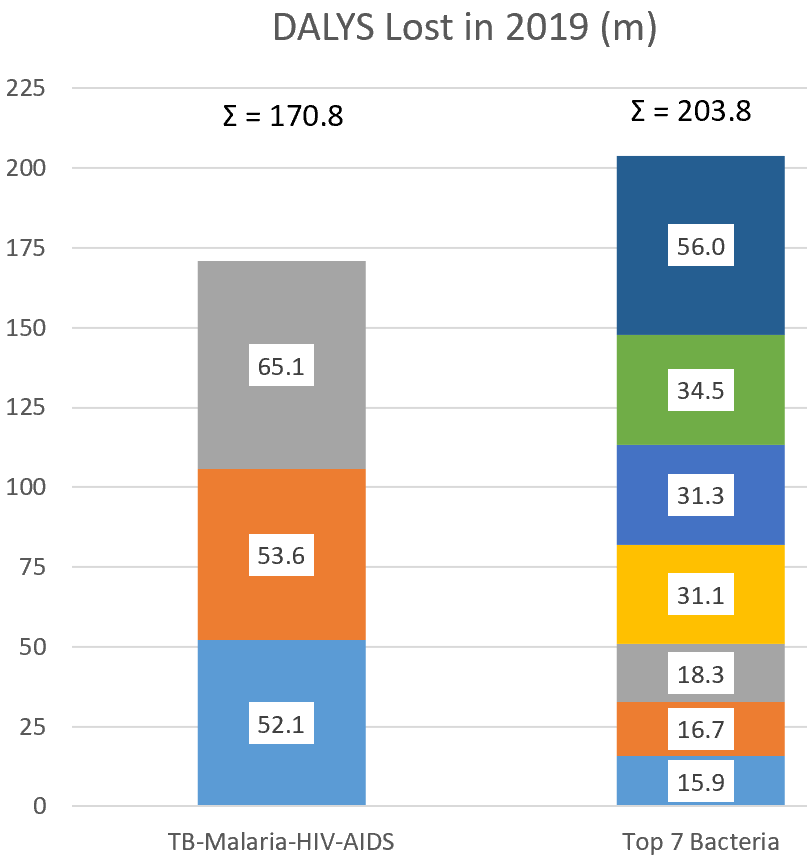
Figure created from data in the paper.
Wow! Death and disability from these 7 bacteria exceed tuberculosis, malaria and HIV-AIDS combined. It’s even more dramatic if you include H. pylori (16.4m): with this, the total for bacteria becomes 220.2 DALYs vs. 170.8 for TB-malaria-HIV-AIDS).
And it is also probably not a surprise to learn that these burdens were distributed unevenly across the globe. The less developed parts of the world bear the greatest load — and children under 5 bear the highest burden no matter where you are in the world:

(above) Figure 1 from Naghavi et al.
Hmm! 28% of the (all-cause) GBD is infectious and 14% is bacterial! Clearly, the work we are doing as a community to create new therapeutic and diagnostic options is critical! And, we also know that the pipeline is not where it needs to be … if you need a refresh on this, start with the 20 Feb 2022 newsletter entitled “New Pipeline Analysis: Cancer Projects Were Funded 17x More Than Antibacterials During 2011-2020.” The title pretty much says it all.
—
What does the mean for the R&D community? Significantly, the ratio of R&D $ to infection impact appears unbalanced. Referencing a 2020 paper entitled “The allocation of US$105 billion in global funding from G20 countries for infectious disease research between 2000 and 2017” by Head et al. (Lancet Global Health), we have these data on US R&D spend by pathogen:
- “genital herpes (ranked) among the top two positions in terms of investment, with $3101 per DALY” but was only “responsible for 0·253 million DALYs”
But…
- “syphilis received the lowest proportion of investment, with only $9 per DALY” but was “responsible for 9·54 million DALYs in 2019.”
- “Similarly, S aureus and Gram-negative bacterial infections (primarily E coli and Pseudomonas spp.) received a relatively low investment in research and development, when considering the associated DALY burden estimated in our study.”
- Per our math, S. aureus had R&D $ of ~$41/DALY, E. coli was ~$28/DALY, and P. aeruginosa was ~$55/DALY.
—
So, what actions are needed to ensure we build the pipeline we need? It’s not going to be an instant fix, but we can note that CARB-X is taking steps to rebalance the R&D spend to deliver infection impact. Following its 2022-2023 omnibus solicitation, CARB-X added several projects to its portfolio that focus on the bacteria noted above (there were also projects for diagnosis and treatment of sexually-transmitted diseases, including gonorrhea):
- Three novel therapeutic programs focused on treating infections caused primarily by S. pneumoniae and S. aureus, importantly with the credentials to offer oral routes of administration for maximum impact for patients and
- Two innovative vaccines targeting E. coli and K. pneumoniae, focused on preclinical proof-of-concept for maternal vaccination to prevent neonatal sepsis.
All told, the projects mentioned here + those coming soon = a financial commitment of ~$14m (post-newsletter addendum: See also the 2023 CARB-X annual report that was released 30 April 2024). And as a further step towards adjusting the funding ratio, the CARB-X funding calls for 2024 target these areas:

Significantly, the focus for Therapeutics is Gram-negative pathogens (WHO and CDC threat list pathogens) and the focus for Prevention is S. aureus and E. coli. For further details, see the CARB-X website and this 6 Mar 2024 newsletter. Similarly, GARDP’s strategy is focused on serious bacterial pathogens (especially Gram-negatives).
So, we are seeing major amounts of push funding directed towards key pathogens. Is this enough? Will we have the pipeline we need? When you recognize the many challenges of innovative R&D (see a summary in the 9 April 2024 newsletter entitled “48,015 → 0: Antibacterial Discovery Is Hard. Really, Really Hard”, it would be be easy to lose hope.
But, the level of energy in the R&D community is extraordinary. Having now funded over 100 projects, some of which are VERY audacious, the CARB-X perspective is that we can make progress. Yes, it will require 6,000 projects at the very earliest stages to yield 6 high-quality innovative therapies … and we are now engaged in making that happen:
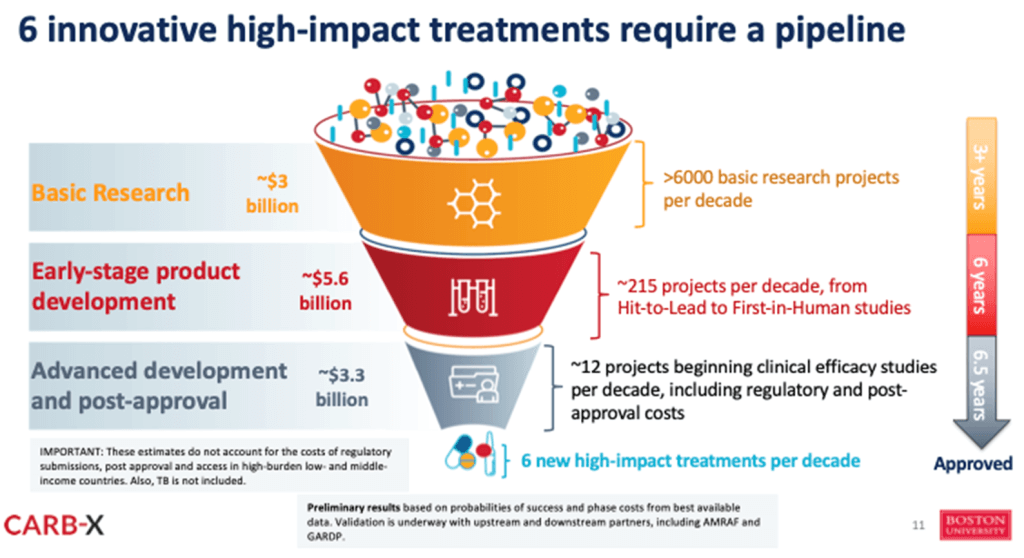
Slide presented by Erin during the ECCMID-GLG AMR pre-meeting symposium on Science and Policy.
Onward! All best wishes, John & Erin
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Erin M. Duffy, PhD | Chief of R&D, CARB-X. emduffy@bu.edu. Follow me on Twitter: @ErinDuffyJ. All opinions are my own.
Footnote: The tally of 350m DALYS (14% of total 2019 DALYs) being due to bacteria is not found in the paper. But, analysis of the line listing in Table 1 permits computation of 352m DALYs in 2019 by totaling the DALYs from these non-tuberculous bacterial causes: Acinetobacter baumannii (16.7m); Aeromonas spp (1.6m); Bordetella spp (pertussis) (11.5m); Campylobacter spp (6.3m); Chlamydia spp (5.6m); Citrobacter spp (1.9m); Clostridioides difficile (2.1m); Diphtheria (0.9m); E. coli (28.5m); E. coli, Enteropathogenic (1.3m); E. coli, Enterotoxigenic (1.5m); Enterobacter spp (11.1m); Enterococcus faecalis (7.0m); Enterococcus faecium (6.0m); Enterococcus, other spp (2.9m); Haemophilus influenzae (5.1m); Helicobacter pylori (16.4m); Klebsiella pneumoniae (31.1m); Klebsiella, Other spp (1.6m); Legionella spp (3.2m); Leprosy (0.0m); Listeria monocytogenes (0.9m); Morganella spp (0.1m); Mycoplasma spp (5.0m); Neisseria gonorrhoeae (0.2m); Neisseria meningitidis (9.4m); Proteus spp (2.7m); Providencia spp (0.1m); Pseudomonas aeruginosa (18.3m); Salmonella Paratyphi (1.6m); Salmonella Typhi (13.2m); Salmonella, Invasive non-typhoidal (14.9m); Serratia spp (4.0m); Shigella spp (7.5m); Staphylococcus aureus (34.5m); Streptococcus pneumoniae (38.1m); Streptococcus, Group A (6.7m); Streptococcus, Group B (11.2m); Syphilis (9.5m); Tetanus (2.6m); Trachoma (0.2m); Vibrio cholerae (6.8m).
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.Current funding opportunities
- CARB-X has open calls that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here for the meeting’s general website. You can’t register (yet) for the 2024 event, but save the date!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
Upcoming meetings of interest to the AMR community:
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 15 May 2024 (in person, New York City, USA; there will be a listen-only webstream): A multistakeholder Hearing for 2024 UNGA HLM on AMR will be held by the Quadripartite Joint Secretariat (QJS-AMR). This is part of the prep for the Sep 2024 High-Level Meeting (HLM) on AMR. Preregistration by 24 April 2024 is required — go here for the registration portal.
- 21-22 May 2024 (hybrid in-person and online, Falls Church, VA, 9a-4p ET both day): 25th PACCARB public meeting. The primary topic is a report to the Secretary of Health and Human Services. Additional topics will cover AMR in conflict zones, the environment, and the voice of the patient.
- As a special event during the meeting, the first of a pair of short films on AMR (18-19 minutes each) will be screened. Collectively titled HOLOBIOME, these films were created with funding from NovoNordisk Foundation and with advisory support from WHO. The films are from the same team (small-r.com) who created the excellent 2015 RESISTANCE movie and cover (i) the story of AMR in a young kidney transplant patient (ii) a sci-fi / documentary hybrid exploring the need for innovation.
- Go here to register for the PACCARB meeting; go here for a preview trailer (~2 minutes) of the short films.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 28-29 May 2024 (in person, Uppsala, Sweden): Uppsala Antibiotic Days, a broad-ranging 2-day program hosted by the Uppsala Antibiotic Center. Go here for details and to register.
- 30-31 May 2024 (face-to-face in Rockville, Maryland as well as online, 8.30-5.30p ET on 30 May, 9-2.40p on 31 May): NIAID-sponsored workshop entitled “Towards realizing the promise of adjunctive immune therapy for invasive fungal infections”. The agenda covers host immunity to invasive fungal infections, immune modulators in the context of fungal infections; and strategies for testing immune modulators as adjunctive therapy. Go here for more details and to register.
- 9-13 June 2024 (in person, Ascona, Switzerland): “New Approaches to Combat Antibiotic-Resistant Bacteria, 2nd Edition” is a Sunday-Thursday residential workshop focused on the deep biology of AMR. Sponsored by NCCR AntiResist (a Swiss National Science Foundation consortium), the scientific program has the feel of a Gordon Conference. Space is limited, so you are encouraged to apply promptly — go here for details.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.