Dear All (wonkish alert … coffee up!),
FDA held a workshop on 25 Sep 2020 on development of inhaled antifungal (AF) therapies with a focus on (i) therapies for allergic bronchopulmonary aspergillosis (ABPA) and severe asthma with fungal sensitization (SAFS), (ii) prophylaxis in lung transplantation, and (iii) adjunct therapy in invasive fungal lung infections (link to FDA webpage with meeting materials).
Aside #1: Note that the focus of this workshop was deliberately NOT on cystic fibrosis (CF): If you are interested in developing drugs for CF, please see this excellent slide deck that Bruce Montgomery shared with me on this topic. You might also want to review the funding opportunities provided by the Cystic Fibrosis Foundation.
Aside #2: A manuscript has been published based on this workshop and is a good summary of the discussion: Jjingo CJ, et al. Food and Drug Administration Public Workshop Summary-Addressing Challenges in Inhaled Antifungal Drug Development. Clin Infect Dis. 2024 Jun 14;78(6):1564-1570. https://doi.org/10.1093/cid/ciad607. PMID: 37802928.
To fully appreciate the insights from the 25 Sep workshop, it is necessary to also be aware of prior material on development of inhaled therapies for bacterial and mycobacterial lung diseases:
- 6 Jun 2017: At the ISAM (International Society for Aerosols in Medicine) congress, FDA’s Sumati Nambiar gave a talk on inhaled antibacterial development (link to newsletter; it links to the ISAM talk as well as a talk on development for HABP-VABP at ASM Microbe).
- 16 Nov 2017 FDA Advisory Committee on Bayer’s inhaled ciprofloxacin for non-CF bronchiectasis (NCFB, link to meeting materials).
- In brief: Mixed results from a pair of big Phase 3 trials (one trial had a significant finding, the other did not), favorable trends were of small magnitude, and patient heterogeneity seemed an issue.
- 11 Jan 2018 FDA Advisory Committee on Aradigm’s inhaled ciprofloxacin for NCFB (link to meeting materials).
- In brief: Similar to the Bayer data, mixed results from a pair of big Phase 3 trials with similar data issues.
- 7 Aug 2018 FDA Advisory Committee on Insmed’s inhaled amikacin for non-tuberculous mycobacterial lung disease (NTM, link to meeting materials).
- In brief: Data from two studies show reduced organism load in sputum but (i) there was no clear clinical benefit based on changes in a 6-minute walk test (one trial had a favorable trend, the other did not) and (ii) the inhaled therapy had a notably higher rate of discontinuation for adverse events such as cough or bronchospasm.
- 8 Apr 2019 FDA Workshop on therapies for NTM lung disease (link to newsletter providing extended notes on that workshop).
- In brief: (i) Clearing NTM from the lungs is hard, (ii) conversion to a negative culture has a weak link to symptoms, and (iii) seeing a clear effect of therapy is confounded by issues such as structural lung disease, baseline heterogeneity, and AEs due to inhaled therapies.
Putting everything together, the key message is that inhaled therapies for pulmonary infections are surprisingly hard to develop. Let’s take the discussion in pieces based on this outline:
- Drug delivery is hard: Inhaled therapies for pulmonary infections sound like they would be a great idea but uniform delivery to the lung is hard (and may not even be possible).
- For the non-invasive syndromes, associated symptoms have many causes as well as a range of severity and durations: For illnesses characterized by exacerbations (NCFB, ABPA, SAFS), the seemingly obvious endpoints of time-to-exacerbation and exacerbation frequency give inconsistent results. For all syndromes, patient heterogeneity creates noise, underlying structural lung disease is not going to be corrected, and other events (e.g., intercurrent viral infections) will further add noise.
- For the non-invasive syndromes, microbiologic endpoints are not the answer: Reduction in organism load does not equate to clinical benefit.
- For the non-invasive syndromes, would a PRO (or two) help the field? What matters is how the patient feels — can we develop suitable patient-reported outcome (PRO) tools?
- Prevention of invasive syndromes in lung transplant is intriguing but prophylaxis is now standard and hence new agents would have to be studied in a non-inferiority design trial.
- Impact of an adjunct inhaled AF in treatment of active infection will be hard to show because concomitant systemic therapy is likely needed. Hence a new agent would need to show superiority over the outcome with well-dosed systemic therapy.
—
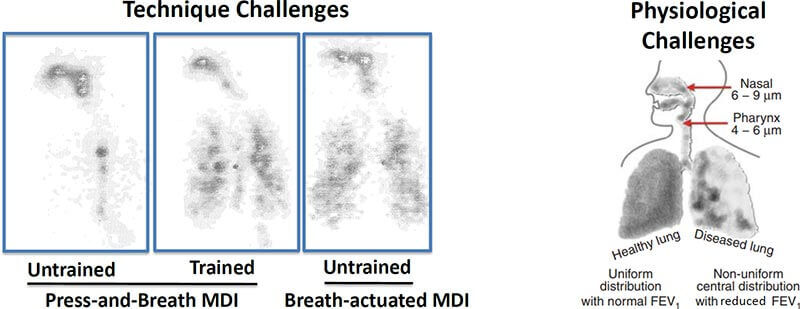
Drug delivery is hard: Although Inhaled therapies for pulmonary infections sound like they would be a great idea, this image from the talk by Dr. Bensman from FDA really puts things into perspective (link to directly download his talk; MDI = Multi-Dose Inhaler). Note at left that even a healthy person can struggle to get consistent distribution — training helps as does as a breath-actuated device but distribution still imperfect. Then at right we have the real problem: airflow in abnormal lungs is (doh!) abnormal. I think it is obvious that areas that really need the drug may well not get it.
—
For the non-invasive syndromes, associated symptoms have many causes as well as a range of severity and durations: For illnesses characterized by pulmonary symptom exacerbations (NCFB, ABPA, SAFS), the seemingly obvious endpoints of time-to-exacerbation and exacerbation frequency have given inconsistent results across trials to date. You can see this for both bacterial infections (links above) as well as for the fungal syndromes (direct link to the talk at the workshop by Denning; it has good listings of prior studies).
A further subtlety that comes out when you read the comments in the FDA briefing books for the inhaled ciprofloxacin studies is that frequency of exacerbation gets confused by the fact that the duration of an exacerbation subtracts from the total at-risk period for an exacerbation … so, there is the counterintuitive possibility that having a single exacerbation will actually reduce apparent exacerbation frequency over any given period of time! For more on this point, go to page 38 in FDA’s BB for the Bayer product and to page 31 in FDA’s BB for the Aradigm product.
Finally, my reading is that all the studies have struggled with signal-to-noise ratio. Patient heterogeneity definitely creates noise, underlying structural lung disease is not going to be corrected by these therapies, and other events (e.g., intercurrent viral infections) will occur. To my eye, this means therapies that produce a real but only modest benefit will struggle to show impact.
As a demonstration of this, the paper Kollef et al. entitled “A Randomized Trial of the Amikacin Fosfomycin Inhalation System for the Adjunctive Therapy of Gram-Negative Ventilator-Associated Pneumonia: IASIS Trial” (Chest. 2017 Jun;151:1239-1246. doi: 10.1016/j.chest.2016.11.026) showed that adjunct inhaled amikacin + fosfomycin reduced organism burden but had no discernable effect on clinical outcome in VAP.
As a counterpoint, the use of organism load as a surrogate marker led to an accelerated approval by FDA for Insmed’s inhaled amikacin for NTM (refractory disease only) that seems likely to have been a one-off that was possible only because there were no approved therapies for that disease. Insmed’s FDA approval (link to press release) requires that they conduct a further randomized trial to demonstrate the clinical benefit. CHMP issued a positive opinion in July 2020 for European approval (link to PR) but we’ll have to await the actual approval to see if it is (as one would guess) conditional on completing a trial similar to that required by FDA.
—
For the non-invasive syndromes, would a PRO (or two) help the field? What matters is how the patient feels — can we develop suitable patient-reported outcome (PRO) tools? This certainly seems like a useful strategy, but it is also one that has some complexity. At a conceptual level, the idea of PRO sounds really good … ask the patient how s/he feels over time and use that as the endpoint.
The tricky thing with PROs, however, is that they typically have 5-10 elements (e.g., pain, ability to do activities of daily living, cough) with each element scored on an ordinal scale using patient scores such as No Symptoms/Limitations – Mild – Moderate – Severe – Disabling. A five-level score like this can be coded as 1-5 (1 = best, 5 = worst).
So, let’s imagine a 5-element PRO and Patient A with a baseline score of 22333 that becomes 11333 after a few weeks. Looks good: the first two scores are better, the others are the same. How about Patient B: 44333 and then 33332 after a few weeks. Again, better … but now who improved the most? Are some elements more important than others? After a while, the mind boggles and you start to really wish for something as simple as “Cured” vs. “Not Cured.”
This is clearly an area where further research needs to be done!
—
Prevention of invasive syndromes in lung transplant is intriguing but the talk by Dr. Husain (direct link) made it clear that prophylaxis is now standard in lung transplant. Hence new agents would have to be studied in a non-inferiority (NI) design trial (link to recent newsletter on the importance of NI designs). I’ve not dug deeply into the literature, but the effect sizes don’t look very large (slides 7-8 from the talk by Dr. Marr, link). This, in turn, could mean that a very large trial is required to show utility of a new agent.
—
Impact of an adjunct inhaled AF in treatment of active infection will be hard to show because systemic therapy is likely needed. Hence a new agent would need to show superiority over the outcome with well-dosed systemic therapy. This is a variation on the problem that we have with non-traditional therapies in general (link to newsletter). Showing incremental superiority over an effective therapy is hard because (i) we go to great lengths in trials to avoid giving ineffective therapy in the control arm and (ii) events unrelated to AF therapy (e.g., progression of malignancy) confound data interpretation.
—
Ok, so we’re now at the end of our tour. It’s been very interesting to review these materials and contemplate at length why such an attractive idea is so hard to pursue in practice. It’s clear that there are patients with serious illness who are not well served by available therapies, but it is equally clear that a lot of work is needed before we’ll have a practical pathway that leads to approval of novel inhaled antifungal and antibacterial products. My thanks to FDA for convening this excellent (albeit sobering) discussion.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- Novo REPAIR Impact Fund closed its most recent round on 31 Jul 2020. Go here for current details.
- 2020 funding rounds for CARB-X have not been announced.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes funders and projects by geography, stage, and more.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- [NEW] ARLG (Antibiotic Resistance Leadership Group, link) is currently open for applications for its 2-year ARLG Fellowship program. The application deadline is 1 Dec 2020; full details are here.
- Finally, you might also be interested in the most current lists of R&D incentives (link) and priority pathogens (link)
Upcoming meetings of interest to the AMR community (most current list is here):
- In case you missed it, the 24 Sep 2020 Bootcamp #1 (“Moving from preclinical to clinical-stage: Challenges & opportunities”) is now available for replay: Get it here. The video for the 8 Oct 2020 Bootcamp #2 (“Exploring safety issues in antimicrobial drug development”) will follow shortly — check back at the current meetings webpage (link) to find it.
- 21 Oct 2020 (online, 9:00-10:30 CEST): GARDP-sponsored webinar entitled “Building better breakpoints: data and methods needed to determine breakpoints for new agents” moderated by Gunnar Kahlmeter. Go here to register.
- 21-25 Oct 2020 (online meeting): IDWeek 2020. Go here for details.
- 26-29 Oct 2020 (online meeting): Annual ESPID meeting (European Society for Pediatric ID, #38)
- 27 Oct 2020 (online, 9a-5p EST): FDA Workshop entitled “Development Considerations of Antimicrobial Drugs for the Treatment of Gonorrhea.” Go here to register.
- 27 Oct 2020 (online meeting): BARDA Industry Day, a discussion of U.S. Government medical countermeasure priorities. Mark your calendar now and watch this website for details.
- 29 Oct 2020 (online, 4-6pm Paris): 5th anniversary ICARe (Interdisciplinary Course on Antibiotics and Resistance) webinar. This fabulous week-long residential course can’t be held this year but Patrice Courvalin is organizing a 2-h anniversary webinar both for former attendees and anybody else who is interested. Speakers will include Patrice as well as Helen Boucher, Gerry Wright, Erin Duffy, and me. Go here to register.
- 5 Nov 2020 (online, 9-10.30am EST) webinar entitled “Aiming in the dark: what happens when disease spreads without diagnosis”, the third webinar in a 4-part series sponsored by Wellcome Trust entitled “AMR in the Light of COVID-19 Webinar Series; From hypothetical to reality: How COVID-19 foretells a world without antibiotics.” Go here to register.
- 16 Nov 2020 (online, 9.30a-4.00p EST): FDA workshop entitled “Potential Approach for Ranking of Antimicrobial Drugs According to Their Importance in Human Medicine: A Risk Management Tool for Antimicrobial New Animal Drugs.” Go here for the FR notice and here for extended details, including registration.
- 17 Nov 2020 (online, 17:00-18:30 CET): GARDP-sponsored webinar entitled “Discovery of new antibacterials using artificial intelligence (computational chemoinformatics)” moderated by Laura Piddock. Go here to register.
- 18-24 Nov 2020 (everywhere): World Antimicrobial Awareness Week. For resources, go here for WHO’s home page for the week. The focus will be on two messages: “Antimicrobials: handle with care” and “United to preserve antimicrobials.”
- 19 Nov 2020 (online, 9-10.30am EST) webinar chaired by Jeremy Knox entitled “Responding to difficult-to-treat infections: Role and responsibilities of governments, researchers, clinicians, industry and patients”, the final webinar in a 4-part series sponsored by Wellcome Trust entitled “AMR in the Light of COVID-19 Webinar Series; From hypothetical to reality: How COVID-19 foretells a world without antibiotics.” Go here to register.
- 26-28 Jan 2021 (online, runs ~7.30a-5.00p Central each day): 4th Annual Texas Medical Center Antimicrobial Resistance and Stewardship Conference. Sponsored by McGovern Medical School, ARLG, and the Gulf Coast Consortia, the agenda includes both poster sessions and keynotes. The call for abstracts closes 18 Dec 2020. Go here for more details.
- 9-12 Jul 2021 (Vienna): Annual ECCMID meeting (#31)
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 3-7 Jun 2021 (Anaheim), ASM Microbe 2021. Go here for details.
- 27 Jun-2 Jul 2021 (Ventura, CA): Gordon Research Conference entitled “Antimicrobial Peptides”. Go here for details, go here for the linked 26-27 Jun Gordon Research Seminar that precedes it.
- 5-21 Aug 2021 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.