Dear All,
Novel antibacterial agents, vaccines, and diagnostics will do little if they are not widely available and used responsibly. CDDEP’s recent report entitled “The State of the World’s Antibiotics in 2021” makes this very clear: “… more people in LMICs (low-middle-income countries) die from lack of access to antimicrobials than from resistant infection.”
Hence, CARB-X has required from the very start that all grant recipients commit to creating a Stewardship & Access Plan (SAP) within 90 days of their product entering pivotal (Phase III or the equivalent for diagnostics) clinical development.
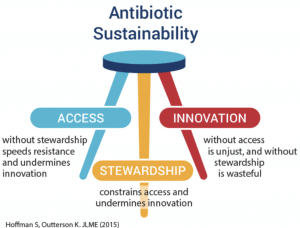
Although this is easy to articulate at a high level (“Please have a plan!”), the best approach to balancing access, stewardship, and innovation has long been a source of debate within the community.
As the SAP would be shared publicly once the product has obtained regulatory approval and would need to cover both Stewardship (appropriate use) and Access (Accessibility, Availability, Affordability, and Acceptability), it’s obviously an ambitious document. But, providing stewardship and access is also not something that the developer can do alone — others also have a role to play.
To support CARB-X grantees as they seek to address this task, a SAP Development Guide has been published today (press release) that provides guidance on meeting the SAP requirement. It is an impressive and thoughtful document that has been created by Wellcome Trust in collaboration with CARB-X, its funding partners, and other interested parties: US Dept of Health and Human Services Office of Global Affairs (DHHS OGA), BARDA, the Global AMR Innovation Fund (GAMRIF), UK Department of Health and Social Care (DHSC), the Bill & Melinda Gates Foundation (BMGF), Wellcome Trust, GARDP, and the Access to Medicine Foundation (ATMF).
The Guide suggests a structure for a SAP and supports this with a detailed list of points to consider (Annex B), a starter list for possible strategies (Annex C) and links to organizations that can provide support and partnering opportunities (Annex D). It is obvious that a lot of thought has gone into this! In particular, the list of plausible strategies in Annex C make this document a strong starting point on what can reasonably be expected of product developers, whether part of the CARB-X system or not.
Tim Jinks, Head of the Drug-resistant Infections Programme at Wellcome Trust said, “The SAP Development Guide represents a new benchmark for what can be expected from companies preparing to market innovative antibiotics, as they should proactively develop robust plans enabling worldwide stewardship and equitable access alongside market entry plans. We hope that these insights inform the wider pharmaceutical, scientific and global health community, as well as CARB-X awardees.”
Impressive! But it is critical to note that responsibility for implementing a SAP does not lie solely with product developers. This point is not explicitly covered in the SAP guide, but many of the actions required can only be suggested by the product sponsor and greater action is also needed from governments, donors and civil society, working with industry, to develop innovative solutions that both accelerate equitable access to new products and slow the spread of bacterial drug resistance.
In particular, delinked Pull incentives are needed both to attract R&D investment and to support the pipeline and the newly approved antibiotics to which the SAP will be applied. On this theme, Thomas Cueni, Director General and key architect of the recently announced AMR Action Fund (see this newsletter for more) said, “With the recent commitment by Industry of ~$1b for the AMR Action Fund that will support Phase 2-3 development and bring 2-4 important new antibiotics to approval by 2030, the pharmaceutical industry is definitely stepping up to meet the challenge of AMR! But, the recent market failures of newly launched antibiotics show clearly that governments need to take action now to implement the delinked incentive models that will sustain these new agents after they are approved.”
While reviewing SAP Development Guide, you should also review the recent report from ATM entitled 2021 Access to Medicine Index. ATMF contributed to the SAP Development Guide and it is exciting to see the momentum that is building. Here is a summary provided by ATMF:
- “The most notable progress is in planning ahead during R&D to make future products accessible: eight companies are developing approaches for systematically ensuring all R&D projects are paired with plans to increase access in poorer countries soon after launch (these have yet to be applied across late-stage projects).
- “The industry has stepped up efforts to understand the outcomes of their access-related activities, and more companies are evaluating initiatives to build local capacity and strengthen health systems than in 2018.
- “All 20 companies have now set specific goals and targets for improving access, and more companies are deploying business models that explicitly include people at the base of the income pyramid.
- “There is also movement in responsible promotional practices, with three additional companies adopting rewards schemes that decouple sales agents’ incentives from sales targets only (now 12 companies).”
Hooray for CARB-X, Wellcome Trust, and their partners! The SAP requirement is a reminder that none of us are safe from AMR until we are all safe! As stated by the IACG AMR (Interagency Coordination Group on Antimicrobial Resistance) in their 2019 report to the UN Secretary-General, “Antimicrobial resistance is a global crisis that threatens a century of progress in health and achievement of the Sustainable Development Goals.”
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- [IMPORTANT UPDATE] Novo REPAIR Impact Fund will open its next global call on 1 April 2021. Go here for current details.
- [NEW] NIAID’s 2021 Broad Agency Announcement for product development is entitled “Development of Medical Countermeasures for Biothreat Agents, Antimicrobial-Resistant Infections an Emerging Infectious Diseases” and is now live with a 24 May 2021 deadline. Research areas include Vaccines, Therapeutics, and Sequencing-Based Diagnostics.
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link)
Upcoming meetings of interest to the AMR community (most current list is here):
- 24 Mar 2021 (online, 2.30-4p CET): GARDP-sponsored webinar entitled “Discovering and developing new treatments for tuberculosis,” moderated by Lydia Nakiyingi. Go here to register.
- [NEW] 25 Mar 2021 (online, 8.30-9.30a EST, 2.30-3.30p CET, 7-8p IST): CDDEP- and WHO-sponsored webinar entitled Vaccines and AMR. The impressive speaker lineup includes Hanan Balkhy (Assistant Director General for AMR at WHO). Go here to register.
- 20-22 April 2021 (online, 1-5p CET): JPIAMR-sponsored workshop entitled “Feeding the Antimicrobial Therapeutics Pipeline.” JPIAMR, the collaborative global effort of 28 countries (much of the EU and also Argentina, Canada, Egypt, Israel, Japan, Korea, South Africa, and Turkey), is organizing a mixture of keynote talks, abstract presentations, and discussion panels designed to encourage collaborative antibiotic R&D. Go here for more details and registration. Abstract deadline is March 17th 2021; no previous JPIAMR funding required.
- 9-12 Jul 2021 (virtual): Annual ECCMID meeting (#31)
- 10-12 May 2021 (virtual): UK-focused Virtual AMR Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free 3-day virtual event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. It will be followed (COVID-willing!) by a face-to-face mission scheduled for 11-15 Oct 2021. Go here for more details.
- 18-21 May 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 24-29 May 2021 (online and in Geneva): ESPID 2021, the 39th Annual Meeting of the European Society for Paediatric Infectious Diseases. Save-the-date announcement is here, details to follow.
- 20-24 June 2021 (Toronto): International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12). Go here for details.
- 20-24 Jun 2021 (virtual, various times): World Microbe Forum sponsored by the American Society for Microbiology (ASM) and the Federation of European Microbiological Societies (FEMS). Go here for more details and to register.
- 27 Jun-2 Jul 2021 (Ventura, CA): Gordon Research Conference entitled “Antimicrobial Peptides”. Go here for details, go here for the linked 26-27 Jun Gordon Research Seminar that precedes it.
- 14-29 Aug 2021 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 11-15 Oct 2021 (physical, somewhere in the UK): UK-focused Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. Go here for more details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students. Bonus feature: For obvious reasons, the course didn’t happen in 2020! But as a celebration of the course’s 5th year, a webinar version was held on 29 Oct 2020: go here to stream it.
- 25-28 Oct 2021 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.