Dear All, Today we have several useful papers to share.
First, OHE (Office of Health Economics, London) have posted a 4-part blog series that provides a succinct summary of the broken economics of antibiotics. The titles are good guides to content:
- Part 1: Why NICE and NHS England are Testing an Innovative HTA and Payment Model to Tackle Antimicrobial Resistance
- Part 2: Value Assessment within the NICE-NHS AMR Pilot
- Part 3: Creating a Healthy Global Market for New Antibiotics
- Part 4: What Does the Antibiotics Market of the Future Look Like?
Focused on the UK’s Netflix subscription model, this series is a great introduction to the broken economics of antibiotics. I liked it so much that I pulled all 4 blogs into a Word document for ease of reference. I particularly liked the discussion of STEDI (newsletter, YouTube video) in the 2nd blog — there is a graphic that shows how the evaluation of the first two drugs in the pilot used only 3 of the 5 STEDI values … and these were used only partially in the assessment of the value of the new agents to the UK:
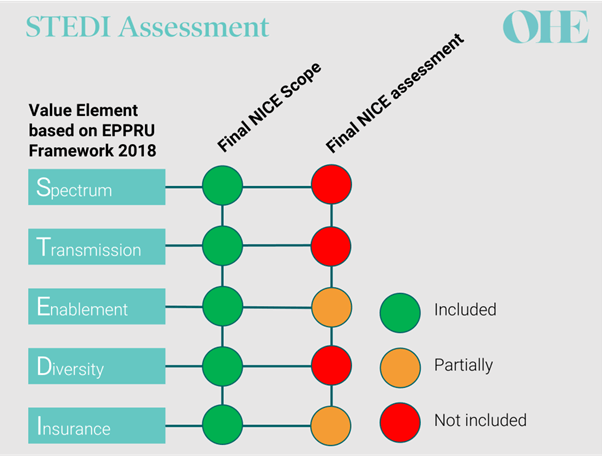
In the figure, note that all 5 STEDI values were in scope for the NICE review but the final assessment was limited in scope: value from the STEDI attributes of Spectrum Transmission, and Diversity was not used at all — all the value came from partial evaluation of Enablement and Insurance!
Very nicely done, Team OHE! I am always in search of new ways to summarize the problem for interested colleagues and it is great to have this very accessible summary; I have added it to the online summary of ways to fund the antibiotic ecosystem.
Second, reviewing the OHE blogs caused me to re-read the recent newsletter on Transferrable Exclusivity Extensions (TEEs) as a Pull tool for Europe … and this, in turn, made me realize that I had failed to mention in that newsletter that the BEAM Alliance had earlier this year released a paper strongly supporting transferrable exclusivity that adds a thoughtful discussion of the value for Europe of a combination of TEEs and Netflix-like subscription models. If you need a review of the Netflix model, Andrew Jack’s 5-minute video explainer is highly recommended. The convergence of these ideas is fascinating!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- Current funding rounds from CARB-X are as described in this newsletter!
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 7 Nov 2022 (virtual, noon-6p GMT): Liverpool School of Tropical Medicine and NW England’s Infection Innovation Consortium (iiCON) are sponsoring an AMR Networking Event (noon-5p GMT; register here) that will be followed by a lecture by Professor Dame Sally Davies on “Antimicrobial resistance: where next?” (5.30-6p GMT; register here). Note there are TWO events here and you need to register separately for both.
- [NEW] 15 Nov 2022 (virtual): WHO webinar entitled “Tracking AMR Country Self-Assessment Survey (TrACSS) Results 2022”. Go here for details. Related to this, there was recently an interesting collection of papers in JAC providing AMR-related data (surveillance, availability of guidelines, availability of drugs) in India, Pakistan, Vietnam, Brazil, Mexico, Türkiye, Russia, Saudi Arabia, and Kuwait.
- 17-20 Nov 2022 (Kuala Lumpur, Malaysia): The International Congress on Infectious Diseases will take place for the first time as a hybrid event. Go here for details.
- 18-24 Nov 2022 (Everywhere!): WHO’s World Antimicrobial Awareness Week is back and happening worldwide. This year, the theme of WAAW is “Preventing Antimicrobial Resistance Together.” Go here for the campaign details.
- 27-30 Nov 2022 (Perth, Australia): 32nd International Congress of Antimicrobial Chemotherapy is the biennial congress of the International Society of Antimicrobial Chemotherapy (ISAC). Go here for details.
- 3-7 Dec 2022 (Banff, Canada): Novel Approaches Against Emerging Antimicrobial Resistance by Keystone Symposia. Go here for details.
- 14 Apr 2023 (Copenhagen, Denmark; 3-6.30p CEST): ECCMID and the Global Leaders Group on AMR will jointly sponsor a symposium entitled “Forging partnerships between science and policy in Antimicrobial Resistance (AMR).” Go here to register.
- 15-18 Apr 2023 (Copenhagen, Denmark): 33rd ECCMID. Go here for details and to register.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.