Dear All: Two updates today.
First, our colleagues at London School of Economics have just published a review of antibiotic innovation incentives that includes a detailed review of some of the currently available sources of funding (Simpkin VL et al., Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps. J Antibiot, 1 Nov 2017).
As the authors noted in a prior paper, there have been more than 50 initiatives in the past decade on this topic! To keep things manageable, this new paper limits itself to initiatives at the multi-lateral, EU, US, and UK levels. This figure from (their Figure 4) gives you a quick sense on the activities they have surveyed:
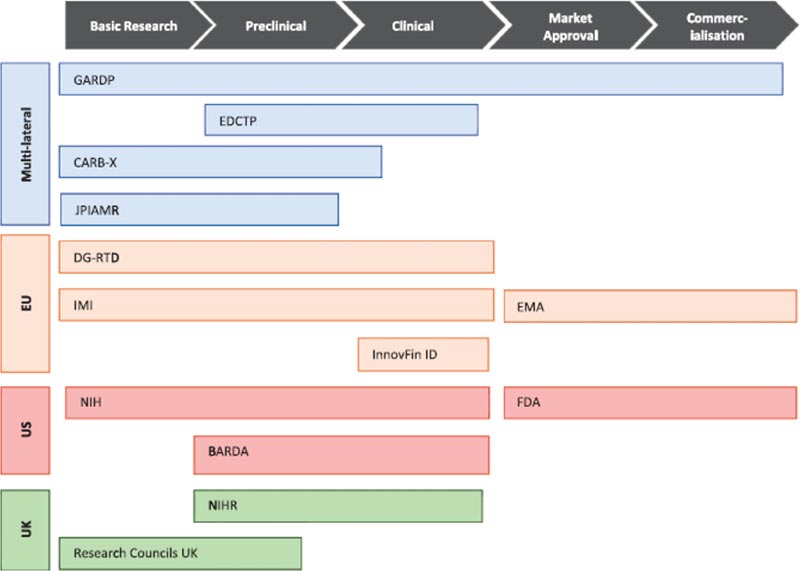
The paper is open access and I encourage you to take a look if you want to be sure you have considered all the sources of funding that might be relevant to your work.
Second, FDA has announced the availability of a draft guidance entitled ‘‘E9(R1) Statistical Principles for Clinical Trials: Addendum: Estimands and Sensitivity Analysis in Clinical Trials.’’ Here is the PDF of the announcement and here is link to the draft guidance. FDA says this about it (lightly reformatted by me for ease of reading):
- The draft guidance was prepared under the auspices of the International Council for Harmonisation (ICH), formerly the International Conference on Harmonisation.
- The draft guidance clarifies, updates, and extends the earlier E9 Statistical Principles for Clinical Trials in two main areas.
- Concerning estimands, it provides a framework for discussion of how the aims of a trial relate to the proposed statistical analysis.
- Concerning sensitivity analysis, it discusses how to use additional analyses to address concerns about the validity of assumptions underlying the main analysis.
- The draft guidance is intended to better align the choice of statistical methods with questions of regulatory importance and to improve the reliability of decisions about and representations of the effects of medical products.
I’m still digesting this one but at its heart is the problem of including important intercurrent events (e.g., change therapy) in the trial’s outcome measures. The term estimand is introduced as the idea of a measurement that includes the population of interest, the endpoint of interest, a specification of how intercurrent events are handled, and a population level summary of the endpoint.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Expert-in-Residence, Wellcome Trust. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://13.43.35.2/blog/
Upcoming meetings of interest to the AMR community:
- 3 Nov 2017 (no specific location): One Health Day
- 6 Nov 2017 (Boston): BAARN (Boston-Area Antimicrobial Resistance Network), Annual Meeting for 2017
- [NEW] 7 Nov 2017 (Washington, DC): FDA VRBPAC (Vaccines & Related Biologic Products) AdComm on the clinical development plan for Pfizer’s investigational Staphylococcus aureus vaccine intended for pre-surgical prophylaxis in elective orthopedic surgical population
- 7-8 Nov 2017 (Washington, DC): BARDA Industry Days
- 11-19 Nov 2017 (Annecy, France): International Course on Antibiotics and Resistance (ICARE)
- 13-19 Nov 2017 WHO’s World Antibiotic Awareness Week
- 15 Nov 2017 (London): BSAC: AMR Market Lounges. Networking day.
- 20 Nov 2017 (week of, China): UK-China Newton Fund Workshop: Antimicrobial Resistance Centre Partnerships Initiative.
- 20 Nov 2017 (London): Early Career Researcher Workshop on Diagnostics for Antimicrobial Resistance.
- 24-27 Nov 2017 (Taipei): 30th International Congress of Chemotherapy & Infection (ICC)
- 12-14 Feb 2018 (Baltimore): ASM Biothreats Conference
- 11-16 Mar 2018 (Ventura Beach): Gordon Research Conference on Antibacterial Discovery
- 21-24 Apr 2018 (Madrid): ECCMID
- 7-11 Jun 2018 (Atlanta): ASM Microbe
- 22-27 Jul 2018 (Bryant University, Smithfield, RI): Gordon Research Conference on Drug Resistance for Cancer, Infectious Disease and Agriculture