This is the first of a 4-part newsletter series. There is a second (28 Sep 2023) newsletter that expands on the EPA concept note, a third (12 Jan 2024) newsletter about ending the use of streptomycin spray on citrus crops, and finally a 4th newsletter (27 Jan 2024) containing some additional resources.
Dear All,
What a week … and I certainly was not expecting this one! … a 4th newsletter in as many days is needed for this Federal Register notice that was published yesterday:
- “Pesticides; Concept for a Framework To Assess the Risk to the Effectiveness of Human and Animal Drugs Posed by Certain Antibacterial or Antifungal Pesticides; Notice of Availability and Request for Comment”: Yes, that’s quite a mouthful … read it slowly!
- The FR notice regarding the “Concept for a Framework”: https://www.federalregister.gov/documents/2023/09/26/2023-20929/pesticides-concept-for-a-framework-to-assess-the-risk-to-the-effectiveness-of-human-and-animal-drugs
- The concept paper describing the approach to developing a framework: https://www.regulations.gov/document/EPA-HQ-OPP-2023-0445-0001
I can hear you thinking: Pesticides? Did you really say pesticides? What do these have to do with AMR? Copying freely from the text in the FR notice, we can take it step by step:
- First, the term “pesticide” in this usage means “any substance or mixture of substances intended for preventing, destroying, repelling, or mitigating any pest…”.
- This definition for pesticide is found in FIFRA, the Federal Insecticide, Fungicide, and Rodenticide Act (7 U.S.C. §136 et seq, 1996).
- So, this definition would include (for example) use of an azole to control fungal growth on plants.
- Next, a pesticide must be registered with the EPA under FIFRA before it can be legally sold or distributed in the United States.
- During the registration process, EPA considers whether the pesticide will cause unreasonable adverse effects on people or the environment.
- Because resistance is considered an adverse effect under FIFRA, the U.S. government is working to develop a structured and coordinated approach to assess and manage these risks.
So, this is really the agricultural equivalent of all the work that has been done to date on reducing veterinary usage (see this 26 Sep 2023 newsletter for any entry point on the veterinary conversation). And, it’s more than agricultural … what about non-agricultural uses such as paint or wood preservation?
With that understanding, the ideas in the FR notice and the concept note become compelling. Here are some excerpts (I’ve drawn variously from the two documents with some light editing for ease of reading):
- “We currently have an incomplete understanding of the factors that contribute to AMR in various settings, including their use in agriculture.
- “Best practices continue to evolve for evaluating the risks to human and animal health posed by pesticides that select for antimicrobial-resistant bacteria or fungi, and substantial uncertainties exist related to this issue.
- “Further, optimal approaches to risk mitigation may be unknown or vary substantially depending on the characteristics of a pesticide and its proposed use.
- “Best practices continue to evolve for evaluating the risks to human and animal health posed by pesticides that select for antimicrobial-resistant bacteria or fungi, and substantial uncertainties exist related to this issue.
- “Through a U.S. government interagency process, the U.S. Environmental Protection Agency (EPA), the U.S. Department of Health and Human Services (HHS), and the U.S. Department of Agriculture (USDA), under the oversight of the White House Executive Office of the President, have developed this concept note.
- The concept note is the first step in creating a framework to improve assessments of potential risks to human and animal health where the use of certain pesticides could potentially result in antimicrobial resistance (AMR) that compromises the effectiveness of medically importanti antibacterial and antifungal drugs.
- “This concept note solicits stakeholder input on the proposed structure for the framework and on potential solutions, research, or mitigation approaches to reduce the spread of AMR.
- “Feedback will then be incorporated, as appropriate, into the draft framework and the final framework, which will be issued at a later date.
- “In particular, input is sought on:
- Ensuring that the framework (see figure below) is appropriately defined and clear to stakeholders,
- The types of pesticides that should be evaluated under the framework, either by class or function,
- The factors that should be considered in determining if a proposed pesticide use constitutes a potential risk to human or animal health due to AMR,
- How to determine which human and animal antimicrobial drugs should be considered ‘medically important’ and how this term should be defined, and
- Mitigation strategies that are currently available to address the risk of AMR developing because of pesticide use.”
Here’s the conceptual framework. It’s drawn from a framework in CVM GFI #152 (“Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effects on Bacteria of Human Health Concern”) and it has three sections: (i) resistance characterization, (ii) risk assessment, and (iii)risk management (including risk mitigation).
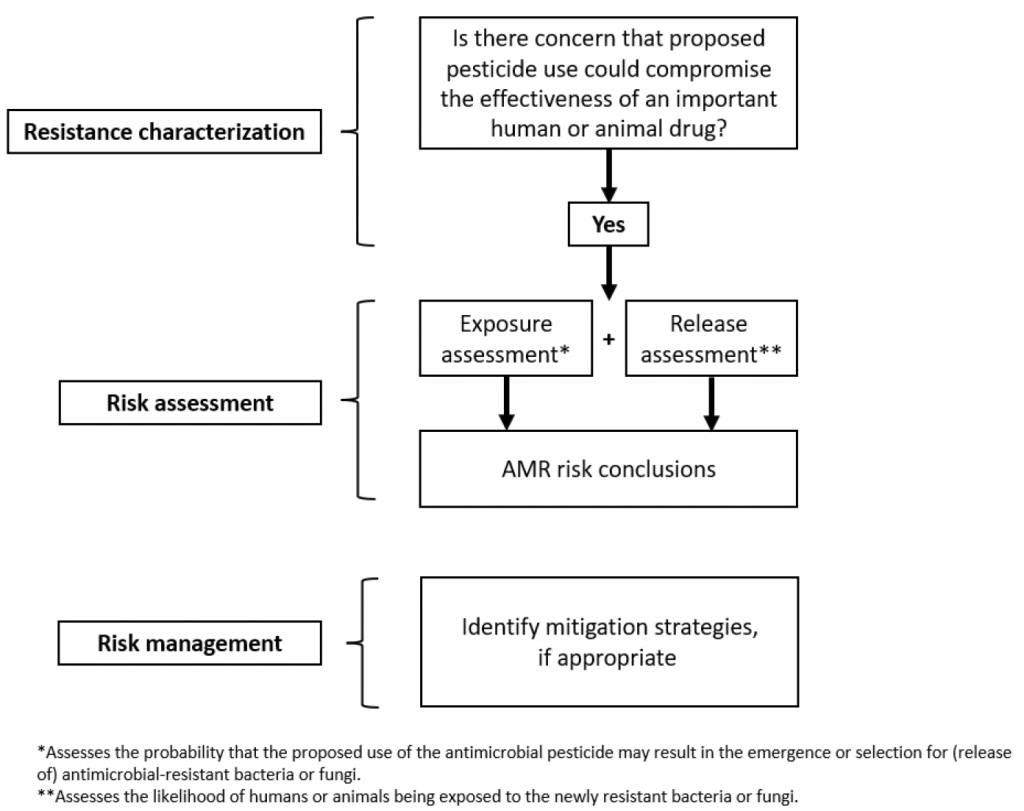
The concept note does not give any specific ideas on the details of such a framework — that’s all for the future. But, the background section of the concept note give an example of how recent evidence indicates that use of azole antifungals in plant agriculture selects for azole-resistant strains of Aspergillus and the note cites Verweij et al. “Dual use of antifungals in medicine and agriculture: How do we help prevent resistance developing in human pathogens?” Drug Resist Updates 2022, https://doi.org/10.1016/j.drup.2022.10088). (Disclaimer: please also know that Verweij et al. discuss two novel mechanism antifungals currently in clinical development as human therapeutics, both of which have agricultural equivalents in development or in use. I am employed by the company that is developing one of these two potential new human medicines.)
The FR notice further expands on the concerns regarding prior examples of the impact of agricultural use in a section entitled “Why is the Agency taking this action?”:
- In the United States, more than 2.8 million antimicrobial-resistant infections occur each year, resulting in more than 35,000 annual deaths.
- Some antibacterial and antifungal pesticides used in agriculture as well as some pesticides used in other settings, belong to the same class as or share mechanisms of action with medically important antimicrobial drugs used in human and veterinary medicine.
- Recent evidence indicates that the use of some antifungal pesticides can select for resistant organisms that pose a potential risk to human and animal health.
- As new pesticides and uses are proposed, the potential exists for these pesticides to select for pathogenic bacteria or fungi that are resistant to medically important antimicrobial drugs, including both FDA-approved drugs and those still undergoing clinical trials.
Wow! It feels like the beginning of a very important conversation. It’s impressive to see the coordination across USG (US Government): EPA + USDA + HHS under the oversight of the White House … wow, again! It may take some time to get to an answer, but well begun is half done.
Your comments on the concept note are sought! Comments should be provided in writing and submitted to EPA Docket ID No. EPA-HQ-OPP-2023-0445 no later than 5:00 pm ET on November 10, 2023.
And (in a news flash that is your reward for reading all the way to the end of this newsletter), I just now learned that President Biden will today announce a $100 million research drive to support the Advanced Research Projects Agency for Health (ARPA-H) in developing new antibacterial agents! Exciting! Addendum: There is a follow-up newsletter that expands on the ARPA-H award
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. Applications are accepted on a rolling basis; go here for all the details.
- The ENABLE-2 consortium has announced a call to support hit-to-lead compound development by researchers at publicly-funded European universities. The call is focused on molecules with the potential to be direct-acting therapies for one or more of the following priority pathogens: ESBL-producing/carbapenem-resistant Enterobacteriaceae (E. coli, K. pneumoniae), P. aeruginosa, A. baumannii, methicillin-resistant S. aureus, or vancomycin-resistant E. faecium. The Call is open continuously, applications are reviewed at intervals, and funding is non-dilutive. Expressions of interest received before 30 Sep 2023 would be considered in November 2023. Applications received after this date will be evaluated in the spring of 2024 (date to be decided). Go to https://www.ilk.uu.se/enable2/apply/ for further details.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- General note: Virtual meetings are easy to attend, but regular attendance at in-person events is the key to networking and deeper insight. My personal favorites for such in-person meetings are marked below as HIGHLY RECOMMENDED and are the BEAM Alliance’s AMR Conference (March, Europe), ECCMID (April, Europe), the ASM-ESCMID Developer’s meeting (September, alternates sides of the Atlantic), and ID Week (October, USA). Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 4 Oct 2023 (Museum of Science, Boston, MA; in person, 11.30a-2.30p ET): Co-sponsored by the Boston’s Museum of Science and the Rijksmuseum Boerhaave (Netherlands), you can attend a luncheon and panel discussion entitled “Celebrating 300 Years of Innovation and Beyond: How van Leeuwenhoek’s Discovery of the Microworld Sparked a Medical Revolution, with a Focus on Antimicrobial Resistance.” Go here for details and to register. I knew about Boston’s MOS but did not know about the exceptional collection of the Rijksmuseum Boerhaave which seeks to “… show what science is all about: curiosity, guts, creativity and perseverance.” Fascinating!
- 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- 11-15 Oct 2023 (Boston, USA): IDWeek 2023, the annual meeting of the Infectious Diseases Society of America. Go here for details and to register. HIGHLY RECOMMENDED.
- 12 Oct 2023 (virtual, 2-3p CET) GARDP-sponsored webinar entitled “Market interventions to improve access to antibiotics for resistant infections.” Go here to register.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.
- 6-7 Feb 2024 (online): Antimicrobial Chemotherapy Conference. This is an annual, free of charge conference that is co-organized by GARDP and the British Society for Antimicrobial Chemotherapy (BSAC). Details to follow — for now, just mark your calendar.
- 6-7 Mar 2024 (Basel,[NEW] 6-7 Mar 2024): Sponsored by the BEAM Alliance, the AMR Conference is now in its 8th year and is consistently an excellent meeting for developers. You can’t register yet but you can mark your calendar and signup for notifications about the meeting. HIGHLY RECOMMENDED.
- 17-22 Mar 2024 (Ventura Beach, CA, in person): Gordon Research Conference (GRC) entitled “New Antibacterial Discovery and Development” with a 16-17 Mar 2024 pre-conference Gordon Research Seminar (GRS) for young doctoral and post-doctoral researchers. An intensive residential meeting, GRCs are highly recommended for networking and deep research insights. Apply here for the GRC and here for the GRS.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details. HIGHLY RECOMMENDED.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here for the meeting’s general website. You can’t register (yet) for the 2024 event, but you can mark your calendar. HIGHLY RECOMMENDED.
