This is the fourth of a 5-part newsletter series. There is an initial 27 Sep 2023 newsletter introducing the EPA concept note, a second (28 Sep 2023) newsletter that expands on the EPA concept note, a third (12 Jan 2024) newsletter about ending the use of streptomycin spray on citrus crops, and a fifth newsletter (11 Oct 2024) about the EPA’s collaborative framework for evaluating the AMR risks of antibacterial and antifungal pesticides.
Dear All,
We’ve had a fascinating series of 3 prior newsletters, all triggered by EPA’s concept note for “Pesticides; Concept for a Framework To Assess the Risk to the Effectiveness of Human and Animal Drugs Posed by Certain Antibacterial or Antifungal Pesticides; Notice of Availability and Request for Comment”:
- The 27 Sep 2023 newsletter taught us the idea of antimicrobials as pesticides.
- The 28 Sep 2023 newsletter told the story of a lawsuit seeking withdrawal of approval to use streptomycin on citrus.
- The 11 Jan 2024 newsletter updated us on the lawsuit (and Yes, streptomycin’s approval must be withdrawn).
As a final follow-up, a co-reader from the One Health world (Neil Vezeau, a veterinarian who is a Technical Consultant and Project Manager for the One Health Commission), shared two further papers that I found very helpful:
- A 2024 review by FAO of all the actions being undertaken globally:
- Title: “Tackling Antimicrobial Resistance in Food and Agriculture”
- Content: A global survey of actions being taken by the Quadripartite, the Global Leaders Group, and the various National Action Plans
- See below my signature for excerpts from this report
- An excellent 2022 paper by Miller, Ferreira, and LeJeune:
- Title: “Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective”
- Content: A detailed class-by-class and compound-by-compound review of use of everything from aminoglycosides to azoles to copper, zinc, and arsenic!
Thanks, Neil, for these additional resources! And if anything else turns up (keep those cards and letters coming!), I’ll add it here.
Onward to the HLM (High-Level Meeting) on AMR at UNGA 2024! All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
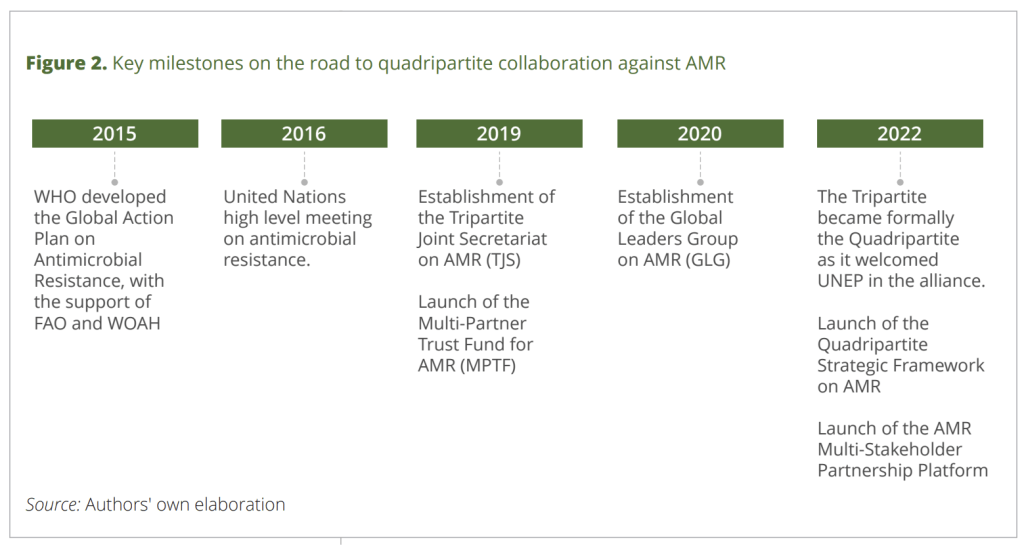
It’s taken years of effort to bring everybody together on this topic! Note how you can see the Tripartite (WHO, FAO, WOAH) becoming the Quadripartite with the addition of UNEP. Here’s some help with the acronymics:
- FAO: Food and Agriculture Organization of the United Nations
- OIE: Office International des Epizooties (is now known as WOAH)
- Quadripartite: FAO + UNEP + WOAH + WHO
- UNEP: UN Environment Programme
- WOAH: World Organization for Animal Health (formerly was known as OIE)
- WHO: World Health Organization
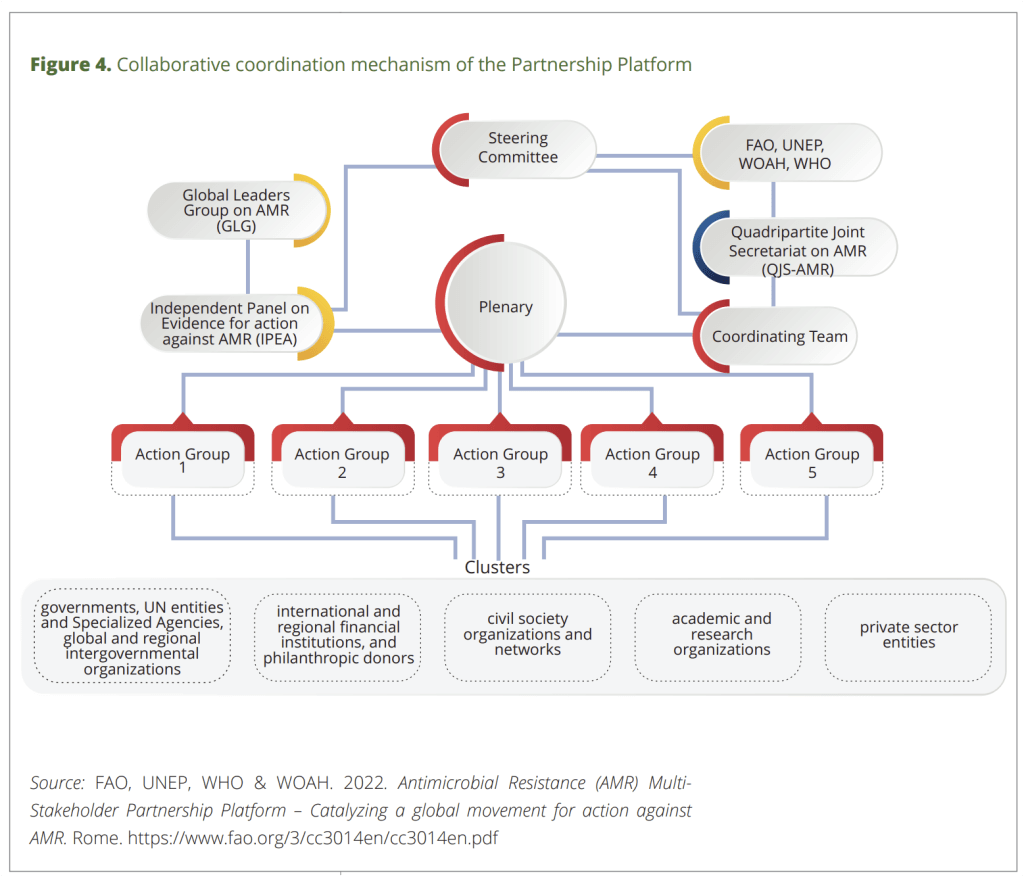
This figure helpfully shows how the GLG AMR (Global Leaders Group, AMR) is connected to the Quadripartite Secretariat and other global actors.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- FDA have released a BAA covering a wide variety of regulatory topics. See this newsletter for general details; also note in particular an RFP for work on urine-specific breakpoints for uUTI. Early concept papers are due 6 Nov 2023; full proposals are due 19 Feb 2024.
- NIAID have a BAA open through 13 Mar 2024 for projects covering vaccines, therapeutics vs. selected pathogens (specific viruses, fungi, and bacteria), and sequencing-based diagnostics. See this newsletter for further details.
- JPIAMR have an AMR Interventions call that is open for pre-applications through 14 Mar 2024. The call covers interventions for both fungi and bacteria. Go here for full details. Note that there is an informational 24 Jan 2024 webinar for applicants.
- ARPA-H have an Open BAA that is accepting applications through 14 March 2024. It is quite wide-ranging in its scope and definitely includes AMR-related projects. See this newsletter for discussion of the BAA and an AMR project that it now supports.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The ENABLE-2 consortium has announced a call to support hit-to-lead compound development by researchers at publicly-funded European universities. The call is focused on molecules with the potential to be direct-acting therapies for one or more of the following priority pathogens: ESBL-producing/carbapenem-resistant Enterobacteriaceae (E. coli, K. pneumoniae), P. aeruginosa, A. baumannii, methicillin-resistant S. aureus, or vancomycin-resistant E. faecium. The Call is open continuously, applications are reviewed at intervals, and funding is non-dilutive. Expressions of interest received before 30 Sep 2023 would be considered in November 2023. Applications received after this date will be evaluated in the spring of 2024 (date to be decided). Go to https://www.ilk.uu.se/enable2/apply/ for further details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 6-7 Mar 2024 (Basel, Switzerland): The 8th AMR Conference 2024. Go here to register!
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here for the meeting’s general website. You can’t register (yet) for the 2024 event, but save the date!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
Upcoming meetings of interest to the AMR community:
- 6-7 Feb 2024 (online): Antimicrobial Chemotherapy Conference. This is an annual, free of charge conference that is co-organized by GARDP and the British Society for Antimicrobial Chemotherapy (BSAC). Go here to register.
- 8 Feb 2024 (in person, Liverpool, UK, 8.30a – 4p): 2024 BioInfect Conference. A full-day AMR conference that includes a keynote from Lord Jim O’Neill (Chairman of the UK AMR Review). Go here for details and to register.
- 14 Feb 2024 (virtual, 8-9a EST): GARDP’s “SECURE: Improving access to antibiotics through new economic models” webinar about the SECURE project. Click here to register.
- [NEW and HIGHLY RECOMMENDED] 15 Feb 2024 (virtual, 8.30a-10.00a EST, 2.30-4p CET, 10.30p-12.00a JST): Entitled “AMR Preparedness Index: 2024 Progress Report”, this will be the launch of a major report by the Global Coalition on Aging (GCOA) and IDSA, and sponsored by IFPMA. Speakers are global (US, EU, Brazil) and feature Dame Sally Davies (UK Special Envoy for AMR) plus a panel moderated by Andrew Jack (Financial Times, creator of a marvelous 5-minute AMR video explainer); the discussion will to cover the new report (it updates the 2021 AMR Preparedness Index), including high-level policy insights from the report in advance of the eagerly anticipated High-Level Meeting on AMR at UNGA 2024 (see 15 Apr 2023 newsletter for background on the HLM). Go here to register.
- 27 Feb 2024 (virtual, 8.30a-9.30a EST): GARDP’s “What does the future look like if pull incentives to support antibiotic R&D are insufficient?” webinar. Go here for details.
- 27 Feb 2024 (in person, New York City, 3-6.30p ET): Hosted by the AMR Industry Alliance (AMRIA), “A Call-to-Action in the Fight Against AMR: Priorities for Progress at the 2024 UN High-level Meeting on AMR” is a symposium (3-5.30p) and reception (5.30-6.30p). Precise details are pending … for now, we are advised to hold the date. I’ll post updates as I receive them … presumably this will also appear on AMRIA’s website.
- 6-7 Mar 2024 (Basel, 6-7 Mar 2024): See Recurring Meetings list, above.
- 17-22 Mar 2024 (Ventura Beach, CA, in person): Gordon Research Conference (GRC) entitled “New Antibacterial Discovery and Development” with a 16-17 Mar 2024 pre-conference Gordon Research Seminar (GRS) for young doctoral and post-doctoral researchers. An intensive residential meeting, GRCs are highly recommended for networking and deep research insights. Apply here for the GRC and here for the GRS.
- 26 Apr 2024 (Barcelona, Spain): ESCMID workshop entitled “Using Data Science and Machine Learning for Infection Science: A Hands-on Introduction.” Click here to register or here for more details.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 9-13 June 2024 (in person, Ascona, Switzerland): “New Approaches to Combat Antibiotic-Resistant Bacteria, 2nd Edition” is a Sunday-Thursday residential workshop focused on the deep biology of AMR. Sponsored by NCCR AntiResist (a Swiss National Science Foundation consortium), the scientific program has the feel of a Gordon Conference. Space is limited, so you are encouraged to apply promptly — go here for details.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.