Dear All,
The AMR Industry Alliance has today released a fascinating and disturbing report. Entitled “Leaving the Lab: Tracking the Decline in AMR R&D Professionals” (press release, report itself), its key message of “Researchers Are Leaving the AMR Field – Even as the Threat Rapidly Grows” is illustrated vividly by the report’s graphics. I’m going to choose 4 to tell the story … here we go!
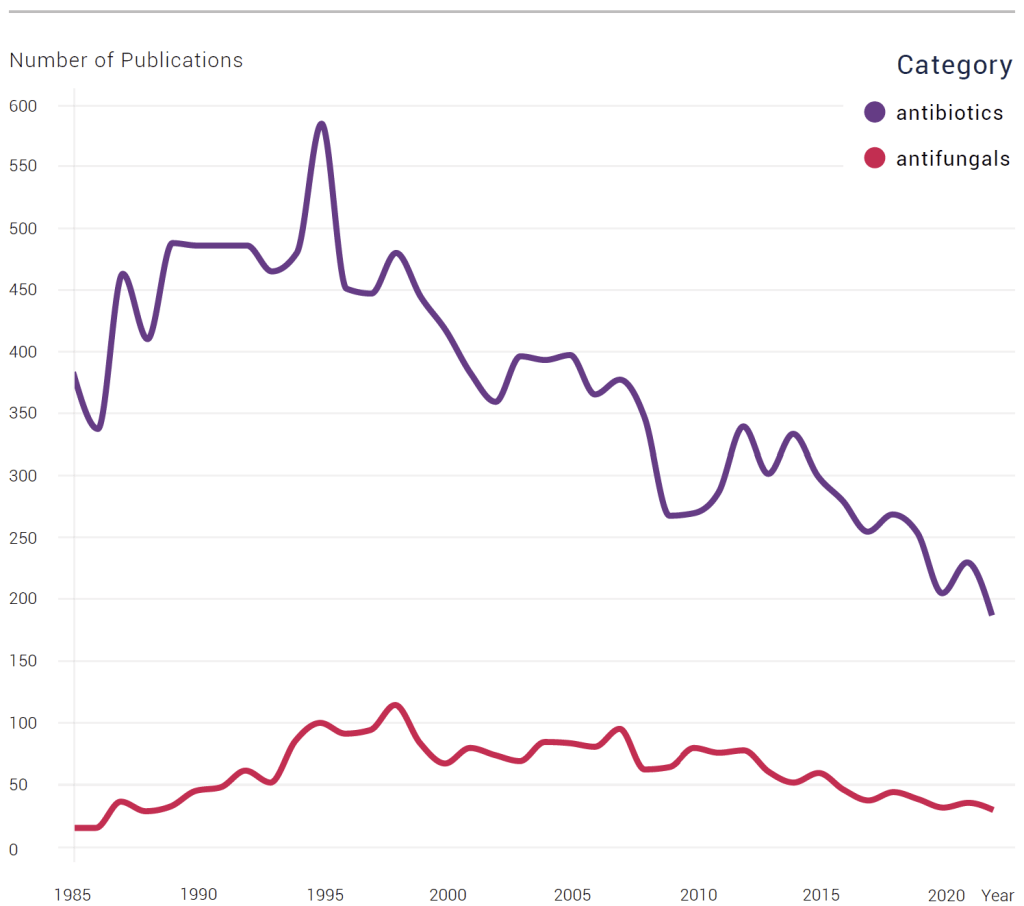
MESSAGE #1: The # of AMR publications has declined over the past 20 years
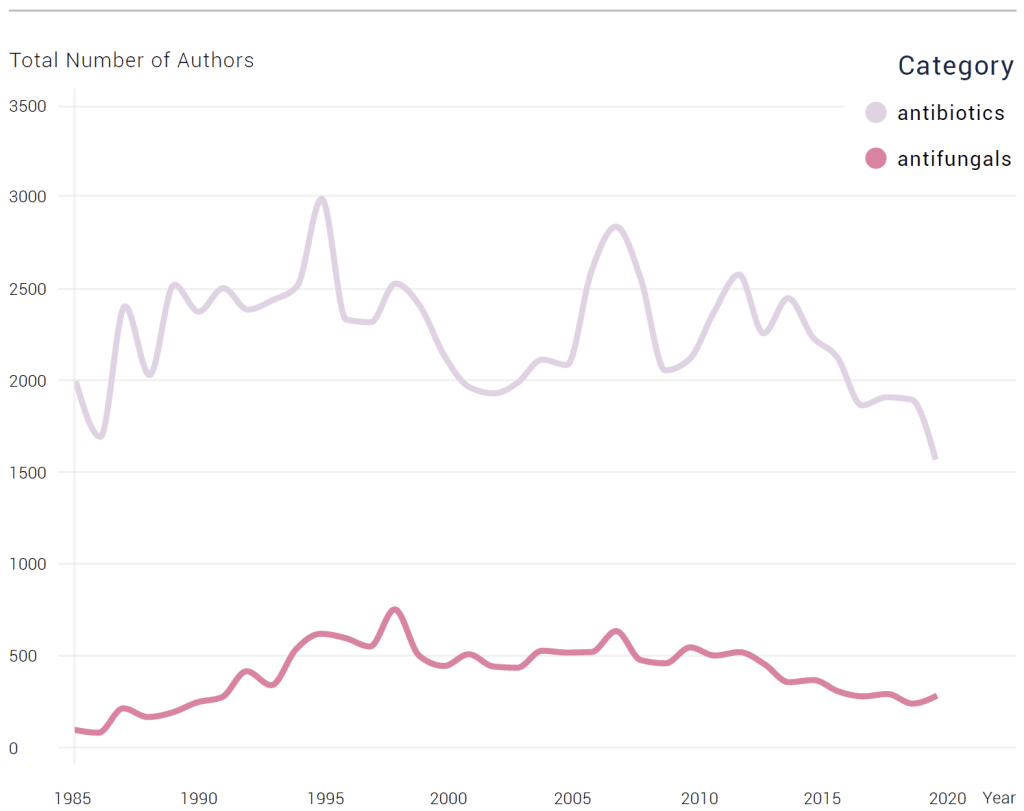
MESSAGE #2: The total # of AMR authors has fallen by almost half since peaking in 1995.
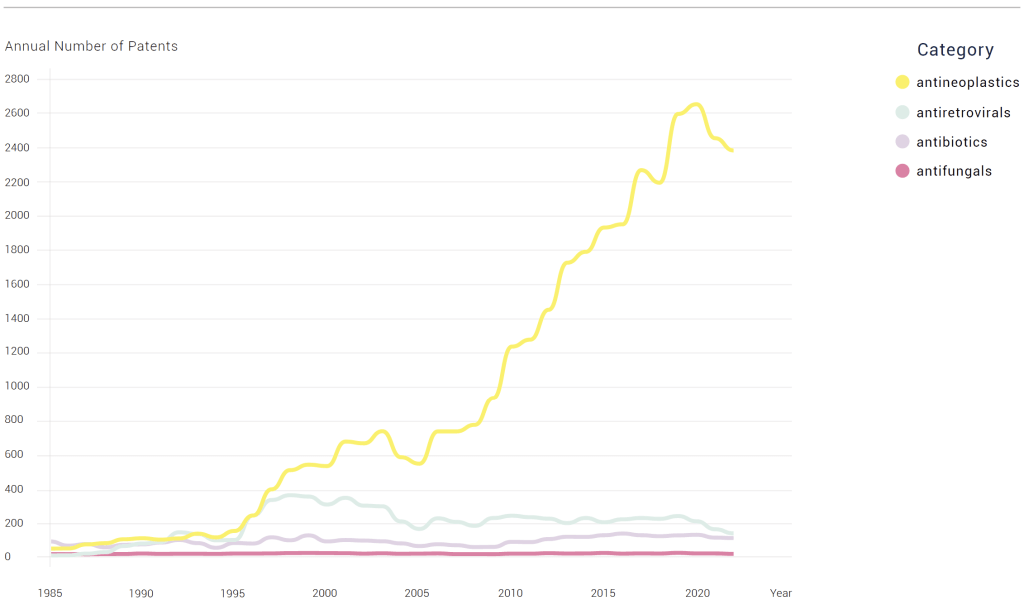
MESSAGE #3: Quoting from the report: “For patents, in 2022, there were approximately 20 times more patents awarded for cancer (2,388) than for antibiotics (115). HIV treatment patent numbers were closer to antibiotics and antifungals, but still higher in every year since 1992.”

MESSAGE #4: Many are called, but few can stay. Based on information from LinkedIn, the authors tracked the career paths of professionals who had been employed by a group of 6 companies that exited the AMR space. These 150 professionals are shown as the dark purple bar at left. Over time, only 12 (8%) of these individuals remained active in AMR R&D.
Companies come and go frequently in R&D (it’s a risky business) but the presumption that attracts researchers is that careers can be built by moving steadily from one company to the next. While that may be true in other areas, it is certainly not true for AMR R&D.
Good parallel reads on this topic are discussed in this 30 June 2020 newsletter and include an analysis by FDA of downward trends (Dheman 2020 plus an accompanying editorial that I wrote with Kevin Outterson), a review of corporate ebb-and-flow showing that the number of active companies peaked in the1990s (Kinch 2014), and a paper showing that antibacterials lag every other type of antimicrobial (Darrow 2020).
This has dire consequences. The report says:
- “… we estimate that approximately 3,000 AMR researchers are currently active in the world (given a range of 1,218 – 4,726).
- “This compares with approximately 29,500 cancer / antineoplastics researchers (12,760 – 46,249) and 3,900 HIV/AIDS / antiretroviral researchers (2,406 – 5,371).
I’ve long had a sense that this was true but seeing it validated by multiple approaches to measuring R&D activity is profoundly disturbing. We simply must give young scientists a good reason to remain in the AMR field! And to do that, we must change the way we pay for the #FireExtinguishersOfMedicine. It’s time for the PASTEUR Act to get passed!
[13 Feb 2024 Addendum]: One more metric regarding limited R&D opportunities would be the tiny number of training courses for those interested in this area. Patrice Courvalin’s excellent ICARe course (an annual, intense, week-long residential program) is the primary global resource and is (to the best of my knowledge) the only soup-to-nuts course of its type. It is supplemented by the focused 2-hour bootcamp sessions run just before the annual ASM-ESCMID meeting and variously by sessions during IDWeek and ECCMID. That’s really it! More is needed!
Sadly yours, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- FDA have released a BAA covering a wide variety of regulatory topics. See this newsletter for general details; also note in particular an RFP for work on urine-specific breakpoints for uUTI. Early concept papers are due 6 Nov 2023; full proposals are due 19 Feb 2024.
- NIAID have a BAA open through 13 Mar 2024 for projects covering vaccines, therapeutics vs. selected pathogens (specific viruses, fungi, and bacteria), and sequencing-based diagnostics. See this newsletter for further details.
- JPIAMR have an AMR Interventions call that is open for pre-applications through 14 Mar 2024. The call covers interventions for both fungi and bacteria. Go here for full details. Note that there is an informational 24 Jan 2024 webinar for applicants.
- ARPA-H have an Open BAA that is accepting applications through 14 March 2024. It is quite wide-ranging in its scope and definitely includes AMR-related projects. See this newsletter for discussion of the BAA and an AMR project that it now supports.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 6-7 Mar 2024 (Basel, Switzerland): The 8th AMR Conference 2024. Go here to register!
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here for the meeting’s general website. You can’t register (yet) for the 2024 event, but save the date!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
Upcoming meetings of interest to the AMR community:
- [Links to prior webinars are available] 7 Feb 2024 (virtual, noon-1.30p CET): WHO-sponsored webinar entitled “4th webinar of the One Health Priority Research Agenda: Intervention Pillar.” As context, the WHO Quadripartite launched its One Health Priority Research Agenda (OHPRA) on 28 June 2023. This new webinar covers one of its five pillars (Transmission; Integrated Surveillance; Interventions; Behavioural insights and change; and Economics and policy). Prior webinars can be found here; presumably the recording from today’s webinar will appear there soon.
- 8 Feb 2024 (in person, Liverpool, UK, 8.30a – 4p): 2024 BioInfect Conference. A full-day AMR conference that includes a keynote from Lord Jim O’Neill (Chairman of the UK AMR Review). Go here for details and to register.
- 9 Feb 2024 (in person, London): “Exploring AI’s impact on AMR,” a workshop co-organised by Imperial’s Institute of Infection, AI Network, NIHR HPRU in Healthcare Associated Infections and Antimicrobial Resistance, and the Centre for Antimicrobial Optimisation (CAMO). Go here for more details and to register.
- 14 Feb 2024 (virtual, 8-9a EST): GARDP’s “SECURE: Improving access to antibiotics through new economic models” webinar about the SECURE project. Click here to register.
- [HIGHLY RECOMMENDED] 15 Feb 2024 (virtual, 8.30a-10.00a EST, 2.30-4p CET, 10.30p-12.00a JST): Entitled “AMR Preparedness Index: 2024 Progress Report”, this will be the launch of a major report by the Global Coalition on Aging (GCOA) and IDSA, and sponsored by IFPMA. Speakers are global (US, EU, Brazil) and feature Dame Sally Davies (UK Special Envoy for AMR) plus a panel moderated by Andrew Jack (Financial Times, creator of a marvelous 5-minute AMR video explainer); the discussion will to cover the new report (it updates the 2021 AMR Preparedness Index), including high-level policy insights from the report in advance of the eagerly anticipated High-Level Meeting on AMR at UNGA 2024 (see 15 Apr 2023 newsletter for background on the HLM). Go here to register.
- 27 Feb 2024 (virtual, 8.30a-9.30a EST): GARDP’s “What does the future look like if pull incentives to support antibiotic R&D are insufficient?” webinar. Go here for details.
- 27 Feb 2024 (in person, New York City, 3-6.30p ET): Hosted by the AMR Industry Alliance (AMRIA), “A Call-to-Action in the Fight Against AMR: Priorities for Progress at the 2024 UN High-level Meeting on AMR” is a symposium (3-5.30p) and reception (5.30-6.30p). For additional information on the event, please contact J.Schumacher@AMRIndustryAlliance.org.
- [HIGHLY RECOMMENDED] 4-5 Mar 2024 (virtual, noon-4.15p ET on both days): “Assessing the Burden and Potential Strategies to Address Antimicrobial Resistance” is a 2-day workshop sponsored by the National Academies that takes a deep dive into what we do and do not know about the costs (clinical and financial) of AMR. Chaired by Jomana Musmar, the deeply knowledgeable Designated Federal Officer who oversees PACCARB, it is no surprise that the agenda is excellent and includes an all-star cast of speakers. Go here to register.
- 6-7 Mar 2024 (Basel, 6-7 Mar 2024): See Recurring Meetings list, above.
- 17-22 Mar 2024 (Ventura Beach, CA, in person): Gordon Research Conference (GRC) entitled “New Antibacterial Discovery and Development” with a 16-17 Mar 2024 pre-conference Gordon Research Seminar (GRS) for young doctoral and post-doctoral researchers. An intensive residential meeting, GRCs are highly recommended for networking and deep research insights. Apply here for the GRC and here for the GRS.
- 25-26 Mar 2024 (In person, London): “Novel Diagnostics for Infectious Diseases,” a 2-day workshop co-organised and funded by Imperial College London’s Institute of Infection, JPIAMR-funded B2B2B Network, London In Vitro Diagnostics Co-operative, NIHR Imperial Biomedical Research Centre, and DIAMONDS consortium. Go here for details and to register.
- 26 Apr 2024 (Barcelona, Spain): ESCMID workshop entitled “Using Data Science and Machine Learning for Infection Science: A Hands-on Introduction.” Click here to register or here for more details.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 9-13 June 2024 (in person, Ascona, Switzerland): “New Approaches to Combat Antibiotic-Resistant Bacteria, 2nd Edition” is a Sunday-Thursday residential workshop focused on the deep biology of AMR. Sponsored by NCCR AntiResist (a Swiss National Science Foundation consortium), the scientific program has the feel of a Gordon Conference. Space is limited, so you are encouraged to apply promptly — go here for details.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.