Dear All (with thanks to Kevin for leading on this newsletter and with a wonkish / long-read alert on this email … settle in for the ride!),
Great news from TEAM UK! As of 12 August, the UK Subscription Program is no longer a pilot but is fully operational with an initial application deadline of 12 Sep 2024. Here are the links you need to follow along:
- The 12 Aug 2024 NHS England webpage that provides the basic announcement
- The Find-A-Tender legal notice: https://www.find-tender.service.gov.uk/Notice/025397-2024
- The detailed procurement documents
- Master website: https://atamis-1928.my.site.com/s/Welcome
- If you click on “View Live Opportunities”, you can search to find the webpage entitled “NHS Antimicrobial Resistance Subscription Model”
- And from here click on the View Documents tab to access the full set of documents starting with Document #1: “Invitation to Tender”
- Rather a bit of digging required here: if you get stuck, there are contact emails readily evident for help
- For context
- The 8 May 2024 NHS England “long read” summary entitled “Antimicrobial products subscription model: guidance on commercial arrangements”
- The 8 May 2024 AMR.Solutions newsletter entitled “New UK 5-year AMR plan: Subscription model details!”
There’s a fair bit to digest if you are going apply, but the key points that everyone needs to know are briefly summarized here with extended details below our signatures:
- The subscription process now covers the entire UK (England, Scotland, Wales, and Northern Ireland) and with this the value bands are higher.
- The payments can be made for up to 16 years, until the UK exclusivity expires (including patents, SPCs, or regulatory data protection).
- The Clinical Technical Evaluation has been thoughtfully updated, giving best-in-world clarity on what will qualify for an antibacterial pull incentive.
- Eligible products include existing and upcoming products.
- This year’s tender has an application deadline of 12 Sep 2024.
- And a few constructive critiques
- The financial stability criteria should better consider both the circumstances and importance of small companies in providing critical antibiotics
- Provisions are needed regarding global stewardship and access
- Provision are needed for increasing value with label expansion [see addendum, below]
27 Aug 2024 addendum: Per a discussion with staff at NICE, products could be moved to a higher (or lower) band if new evidence emerges. The sponsor could submit new efficacy data at/after 3 years in the subscription model; NICE could trigger a review sooner if safety issues arise.
Woot, woot! Wow! Our critiques not withstanding, it is marvelous to see this process become business as usual! MANY THANKS TO TEAM UK for their WORLD-LEADING WORK on Pull Incentives!
It truly is the case that we’ve pushed as hard as we can and and now we need to start pulling via the PASTEUR Act in the US, the different (and potentially complementary) proposals of pull incentives from the European Commission, as well as the ideas/pilots from Japan, Canada, Italy, and Switzerland.
Onward! All best wishes, Kevin and John
Kevin Outterson, JD, Professor of Law, Boston University & Executive Director, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of its funders) @koutterson
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
EXTENDED DETAILS, POINT-BY-POINT
1. The subscription process now covers the entire UK (England, Scotland, Wales, and Northern Ireland) and with this the value bands are higher:
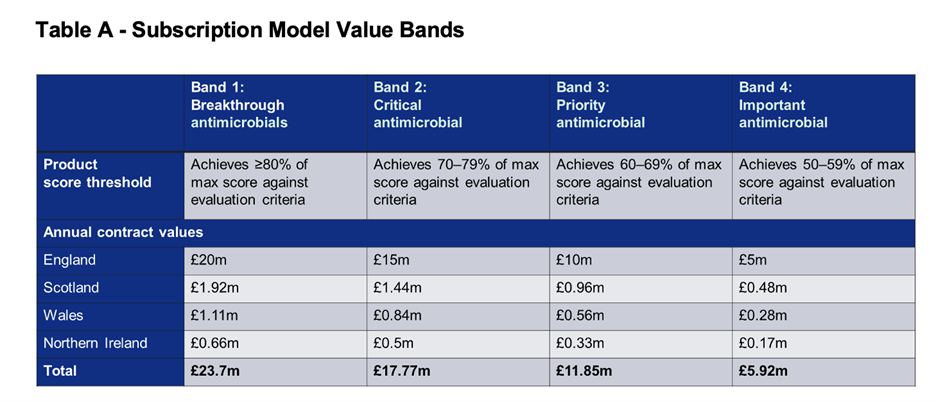
As you can see, the highest possible value is GBP 23.7m ($30.6m at current exchange rates). As the total budget allocated for the entire program is up to £100 million per year, the UK is certainly paying its “fair share” for antibacterial innovation (see also our 2023 article on “fair share”).
2. The payments can be made for up to 16 years, until the UK exclusivity expires (including patents, SPCs, or regulatory data protection).
3. The Clinical Technical Evaluation has been thoughtfully updated, giving best-in-world clarity on what will qualify for an antibacterial pull incentive. The details are found in Document 6 (“Clinical_Technical_Evaluation criteria”) and we highlight 2 key elements:
3.a. The Product will be scored against the criteria below, with the weights shown below. The criteria are in 3 groups: (1A-D) Relative effectiveness and unmet clinical need (45%) of the overall value, (2A-F) pharmacological benefit (25%), and (3A-G) Health system benefit (30%):
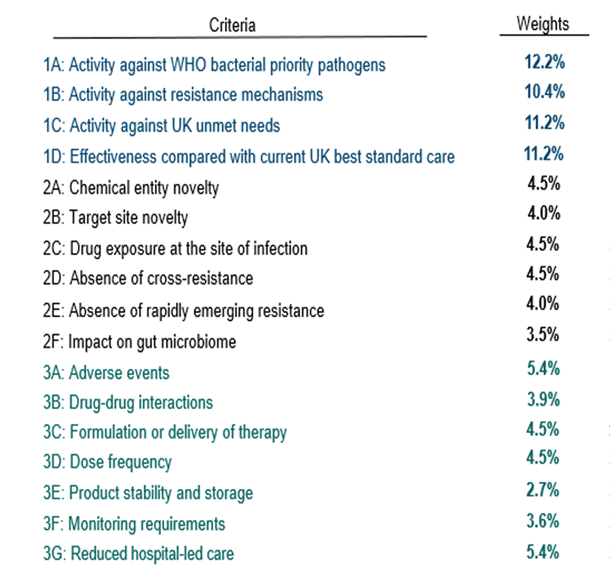
3.b. Each of these factors is explained in the text of Document 6, including the types of evidence that will be accepted. For example, the extended rules for Criteria 1A show how the awarded points follow the WHO Priority Bacterial Pathogen List to vary by pathogen (and these rules also make clear the focus on bacterial pathogens; the pathogens from the fungal PPL are not in scope for this tender):
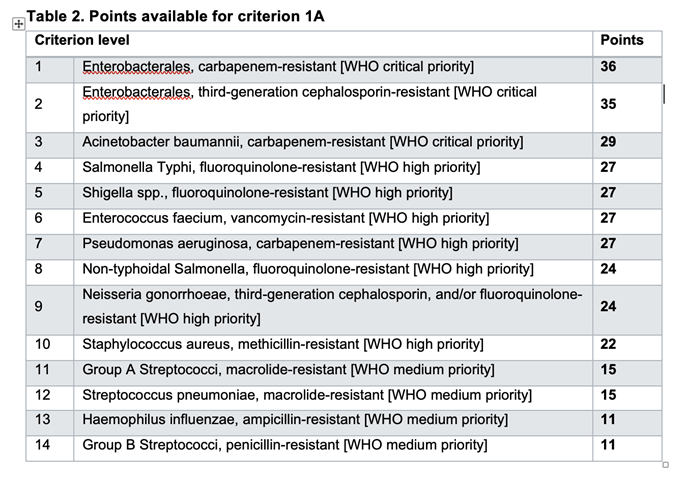
The technical criteria in document 6 are required reading for anyone developing an antibiotic which might want a UK subscription.
4. Eligible products include existing and upcoming products. Here are the eligibility rules:
- “an antimicrobial with a license for the UK and a UK launch where the marketing authorisation has already been granted or is likely to be granted before 31 July 2025 and
- “the Licensed Indication(s) include one or more pathogens on the WHO critical priority pathogen list 2024 against which the antimicrobial is active.
- “The product should also be within the period of the exclusivity defined either by the patent, supplementary protection certificate or regulatory data protection and
- “have a minimum remaining twelve (12) months before the date of loss of exclusivity from the date of commencement of the contract”
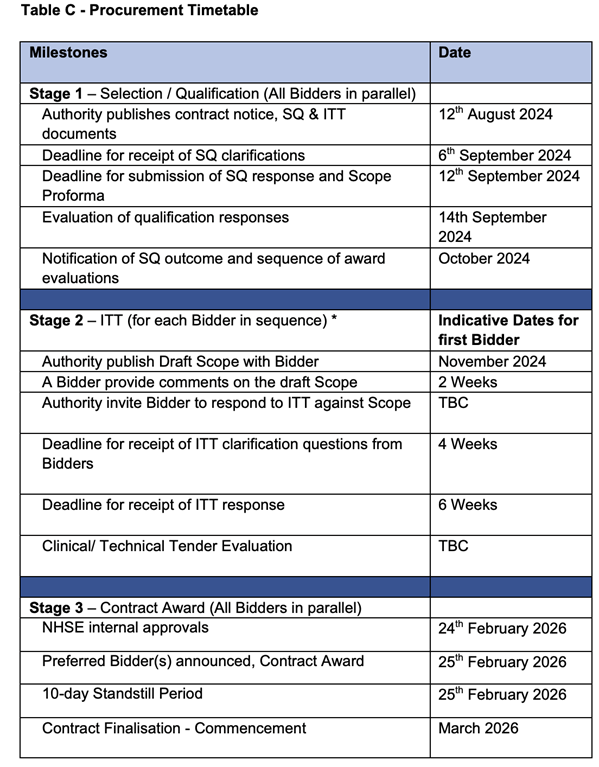
5. Don’t miss the bus! The application timeline for the next round of subscriptions is very short: 12 September (one month from publication, see time table below). The expectation is that the tender will be repeated annually with the proviso that its terms might be updated (from the long read webpage, we have this: “The documentation will include the eligibility and the award criteria. These will be reviewed each year before notice of the tender is given.”)
CONSTRUCTIVE CRITIQUES, POINT-BY-POINT
- We would like to see the UK modify Document 3, “Guide for assessing financial standing.”
- In a normal procurement process, governments want to ensure that the counterparty is financially sound.
- For antibiotics, especially in small companies, pull incentives like the Subscription are also designed to rescue financially struggling companies. It make little sense to apply the standard financial scoring methodology to small antibacterial biotechs.
- It would be akin to requiring proof of financial solvency prior to receiving disaster assistance after a storm.
- This issue was raised in the comment process on the program: In the pilot stage of the Subscriptions, some small company applicants found this to be a formidable barrier.
- This might force small companies to partner with larger, more solvent, companies in order to apply to UK. That might be a good thing, but the decision should be made on business grounds, not the standard language of a procurement agreement.
- Further, the company gets a lower score if this contract is a substantial portion of their global revenue and if they have a bad D&B score.
- Rather than this, we think that we want small companies with no other revenue yet to apply to G7 pull incentives. They need 22 points to pass this financial check.
- We want to hear from any small companies who have a product UK might want, but who miss this pass/fail financial hurdle.
- In a normal procurement process, governments want to ensure that the counterparty is financially sound.
- We would like to see provisions added regarding global stewardship & access.
- The UK has a clear opportunity to lead here by imposing reasonable contractual conditions that support global stewardship and access.
- Shionogi’s license of cefiderocol to GARDP is a good model for this (25 Sep 2023 newsletter).
- This would be a great opportunity to reinforce the ideas from the Stewardship & Access Guide created by CARB-X, Wellcome, and partners (22 March 2021 newsletter).
- No provisions were found allowing the selected drug to be rescored upward after a label expansion or other evidence of greater clinical utility.
- We would like to see processes that support label expansions.
- The GHIT Fund has announced its 21st Request for Proposals for its Hit-to-Lead Platform to support drug discovery and development process to address Malaria, Tuberculosis, Chagas disease, and Visceral leishmaniasis. Go here for the RFP: GHIT-RFP-HTLP-2024-002. The deadline is 30 Aug 2024.
- The AMR Industry Alliance have announced the 2024 edition of their ongoing annual series of stewardship prizes. Applications for innovative approaches to AMR stewardship are sought from public, private or not-for-profit health care organization or institution operating in an LMIC. This year’s deadline is 1 Sep 2024. Go here for details.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X has open calls at intervals that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to register!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Go here for details.
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
Upcoming meetings of interest to the AMR community:
- [HAS HAPPENED; NOTEWORTHY VIDEO]: 22-23 May 2024 meeting of PACCARB. If you were intrigued by the 23 July 2024 newsletter entitled “Conflict-Borne XDR Superbugs”, you should note that PACCARB devoted its 23 May 2024 morning session to the topic of combat-related drug resistance. See page 3 of the agenda and then follow along in the video on YouTube.
- 22 Aug 2024 (virtual, 11a-12.30p EDT, 5p-630p CEST): GARDP REVIVE Webinar entitled “Exploring non-traditional antimicrobials: Insights from three cases.” Go here for details and to register. If non-traditional approaches interest you, please do be sure to review the challenges that are raised in the papers discussed in the 6 Aug 2019 newsletter entitled “Non-Traditional Antibiotics: A Pipeline Review And An Analysis Of Key Development Challenges.” Developing non-traditional products is MUCH harder than you might expect … it is important to know the issues!
- [NEW – DON’T MISS IT] 28 Aug to 28 Sep (Off-Broadway, New York City, the Alice Griffin Jewel Box Theatre): Lifeline, the musical story of Sir Alexander Fleming’s discovery of penicillin. Previously entitled The Mould that Changed the World, the musical is a two-time Edinburgh Festival Fringe sell-out (2018 and 2022) and has toured to London, Glasgow, Atlanta and Washington DC (2022). This 5-week run in NYC is timed to be in support of the High-Level Meeting on AMR (HLM AMR) during UNGA 2024. Go here for a blurb and here to book your tickets!
- [NEW – DON’T MISS IT] 9 Sep 2024 (9a-4p ET, in-person or virtual): FDA have announced an AMDAC (Antimicrobial Drugs Advisory Committee) meeting that will discuss “new drug application 213972, for oral sulopenem etzadroxil/probenecid tablets consisting of 500 milligrams (mg) sulopenem etzadroxil and 500 mg probenecid, submitted by Iterum Therapeutics US Ltd., for the proposed indication of treatment of uncomplicated urinary tract infections caused by designated susceptible bacteria in adult women 18 years of age and older.” Try to make time to listen to this … every FDA Advisory Committee is a master class in regulatory thinking! Go here for the Federal Register notice and here for the meeting’s webpage: materials are typically posted 2 days before the meeting.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- [NEW] 24 Sep 2024 (in person, 7.45-10a ET, New York City): Breakfast meeting entitled “Advancing Together: Securing the Global AMR Agenda by Harnessing the Collective Strength of Multi-Sector Partnerships”, sponsored by bioMèrieux, The Wellcome Trust, The American Society of Microbiology, and the Republic of Malawi. This occurs two days before the 26 Sep 2024 UNGA HLM on AMR. Go here to register.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- [NEW] 16 Oct 2024 (virtual and in-person, 10a-1p ET): FDA’s Rare Disease Innovation Hub, in collaboration with the Reagan-Udall Foundation will discuss how the recently announced Rare Disease Innovation Hub can engage and prioritize its work. This may seem somewhat remote, but could this have implications for rare infections? Hmm! Attend if you can! Go here for the meeting’s webpage.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.