Dear All,
Just in time for the United Nations General Assembly High-Level Meeting on AMR, the team behind the global burden of disease (GBD) estimates which we’ve discussed previously has released an excellent new report! Here are the links you need:
- The new report: “Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050” by the GBD 2021 Antimicrobial Resistance Collaborators, Lancet, 16 Sep 2024, DOI: https://doi.org/10.1016/S0140-6736(24)01867-1.
- The accompanying editorial: “Global burden of antimicrobial resistance and forecasts to 2050” by Samuel Kariuki, Lancet 16 Sep 2024, https://doi.org/10.1016/S0140-6736(24)01885-3.
- 20 Jan 2022 newsletter: “#AMRSOS! GRAM report: “at least 1.27m deaths/year directly attributable to AMR” discussing the first big paper on the GBD for AMR.
- 29 Apr 2024 newsletter: “R&D Implications: Global Burden of Disease is 28% Infectious!” discussing a further analysis showing that 28% of GBD is infectious and14% is bacterial!
- And there is a lot of media coverage: Washington Post and CNN, just to name a few.
This is a substantial paper and you’ll need to make time to digest it. If the GBD analyses are new to you, I suggest you start with #3 above as that newsletter explains the ideas behind the GBD project.
In brief, this new paper analyzes trends from 1990 to 2021 and then projects trends through 2050. Numerically, the punch line is in the title of this newsletter: AMR-attributable deaths will globally total 39m across the period 2025-2050. But, I think a better summary of this is visual. Let’s first consider a global view of AMR mortality rates in 1990 (above) and 2050 (below), with darker red = more deaths:
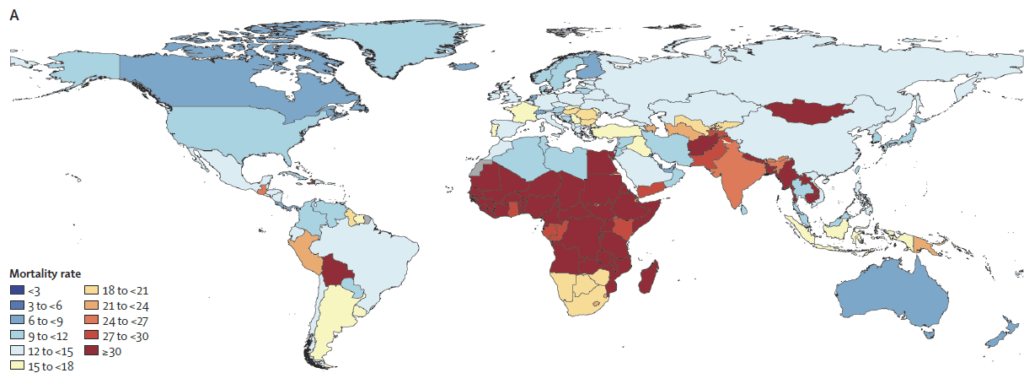
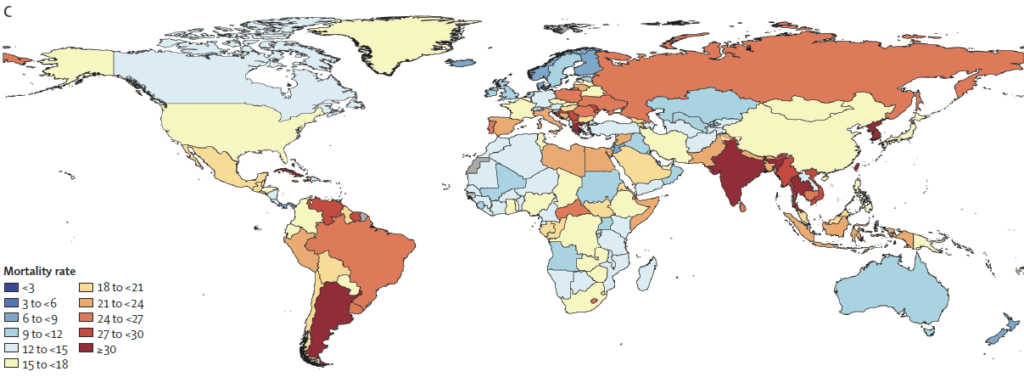
We can then consider pairwise images that zoom in on selected regions (1990 above, 2050 below): Caribbean and Central America, Persian Gulf, Balkan Peninsula, Southeast Asia, West Africa, and Northern Europe:
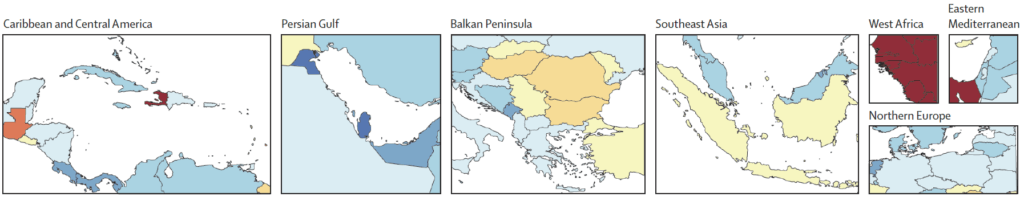
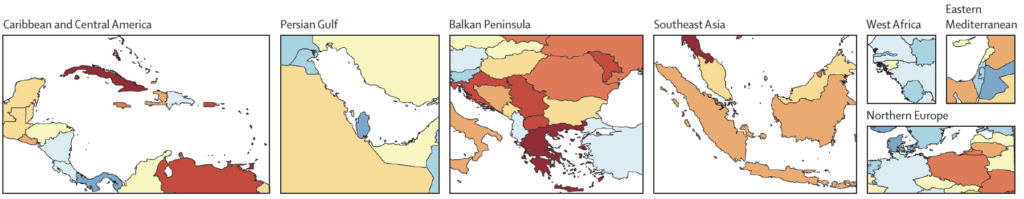
Key messages are:
- Globally, AMR-attributable mortality will increase if we do not take action.
- The rate of increase is uneven!
- Mortality is decreasing in children under 5 (yes, decreasing)
- But mortality is increasing in the older population, with an 80% increase forecast in those over age 70!
- This follows from the huge impact of vaccination and WASH (Water, Sanitation, and Hygiene) on infection rates in children vs. the increasing complexity of a growing older population.
- The net effect is “increasing burden from AMR in the coming decades, with an estimated 69·6% increase in global deaths attributable to AMR and 67·0% increase in deaths associated with AMR between 2022 and 2050.“
- Sadly, this paper concludes “that without additional measures we will fail to hit the 10% reduction in AMR mortality proposed in the 10-20-30 by 2030 target.”
- This is reference to the 10-20-30 target proposed in May of this year … see the superb series of papers discussed in 23 May 2024 newsletter entitled “Lancet: 10-20-30 targets to address AMR by 2030“
Lest you think that you are safe because you might not currently be in one of the dark red countries on the map–think again. In our globally connected world, no one is safe until we all are safe. Sobering … but indeed exactly the kind of evidence that precipitates action!
If you want to read a bit more deeply, look below my signature for an annotated version of the Findings from the abstract. Extraordinary stuff!
With thanks to the team behind the amazing Global Burden of Disease project and also with thanks to all of you are acting to avert this looming disaster, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Annotated elements from the abstract of the paper:
Methods: We estimated all-age and age-specific deaths and disability-adjusted life-years (DALYs) attributable to and associated with bacterial AMR for 22 pathogens, 84 pathogen–drug combinations, and 11 infectious syndromes in 204 countries and territories from 1990 to 2021.
Findings
- “In 2021, we estimated 4·71 million (95% UI 4·23–5·19) deaths were associated with bacterial AMR, including 1·14 million (1·00–1·28) deaths attributable to bacterial AMR.
- “Trends in AMR mortality over the past 31 years varied substantially by age and location.
- “From 1990 to 2021, deaths from AMR decreased by more than 50% among children younger than 5 years yet increased by over 80% for adults 70 years and older.
- “AMR mortality decreased for children younger than 5 years in all super-regions,
- Why? From the body of the paper:
- “Much of this reduction is due to a decrease in drug-resistant S pneumoniae and in pathogens commonly spread through faecal–oral transmission (eg, Salmonella, Shigella, and enteropathogenic or enterotoxigenic E coli).”
- “This decline coincides with widespread vaccination efforts and improved access to WASH and serves as an important reminder to the global health community and policy makers that infection prevention might be a highly effective intervention in reducing AMR burden.”
- … “whereas AMR mortality in people 5 years and older increased in all super-regions.
- !!! Why is this? From the body of the paper:
- “First, the rising AMR and sepsis mortality in older adults has been masked by traditional global health metrics.
- “Second, interventions that are effective in younger age groups, such as vaccines, might be less effective in older adults.
- “Third, older adults are more likely to have adverse effects from specific antimicrobials; as resistance increases, the use of more toxic second-line and third-line treatment options is necessitated, which might not be tolerated in older populations.
- “Fourth, adults are more likely to have comorbidities that lead to immunodeficiencies and increase the risk of opportunistic infections.”
- “For both deaths associated with and deaths attributable to AMR, methicillin-resistant Staphylococcus aureus increased the most globally (from 261 000 associated deaths [95% UI 150 000–372 000] and 57 200 attributable deaths [34 100–80 300] in 1990, to 550 000 associated deaths [500 000–600 000] and 130 000 attributable deaths [113 000–146 000] in 2021).
- “Among Gram-negative bacteria, resistance to carbapenems increased more than any other antibiotic class, rising from 619 000 associated deaths (405 000–834 000) in 1990, to 1·03 million associated deaths (909 000–1·16 million) in 2021, and from 127 000 attributable deaths (82 100–171 000) in 1990, to 216 000 (168 000–264 000) attributable deaths in 2021.
- “There was a notable decrease in non-COVID-related infectious disease in 2020 and 2021.
- “AMR mortality decreased for children younger than 5 years in all super-regions,
- Going forward: “Our forecasts show that an estimated 1·91 million (1·56–2·26) deaths attributable to AMR and 8·22 million (6·85–9·65) deaths associated with AMR could occur globally in 2050.
- “Super-regions with the highest all-age AMR mortality rate in 2050 are forecasted to be south Asia and Latin America and the Caribbean.
- “Increases in deaths attributable to AMR will be largest among those 70 years and older (65·9% [61·2–69·8] of all-age deaths attributable to AMR in 2050).
- “In stark contrast to the strong increase in number of deaths due to AMR of 69·6% (51·5–89·2) from 2022 to 2050, the number of DALYs showed a much smaller increase of 9·4% (–6·9 to 29·0) to 46·5 million (37·7 to 57·3) in 2050.
- From the body of the paper: “Cumulatively from 2025 to 2050, our reference scenario forecasts 39·1 million (33·0–46·0) deaths attributable to AMR”
- “Globally and in every super-region, AMR deaths in children younger than 5 years are decreasing, most pronounced in absolute numbers in sub-Saharan Africa and south Asia.
- “For deaths of people 70 years and older, we forecasted an increase from 2022 to 2050 in every super-region.
- And if take action: “Under the better care scenario, across all age groups,
- “92·0 million deaths (82·8–102·0) could be cumulatively averted between 2025 and 2050, through better care of severe infections and improved access to antibiotics, and
- … “under the Gram-negative drug scenario, 11·1 million AMR deaths (9·08–13·2) could be averted through the development of a Gram-negative drug pipeline to prevent AMR deaths.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X has open calls at intervals that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 17-20 Sep 2024 (Porto, Portugal; virtual attendance is possible): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to register!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Go here for details.
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
Upcoming meetings of interest to the AMR community:
- [ONGOING – DON’T MISS IT] 28 Aug to 28 Sep (Off-Broadway, New York City, the Alice Griffin Jewel Box Theatre): Lifeline, the musical story of Sir Alexander Fleming’s discovery of penicillin. Previously entitled The Mould that Changed the World, the musical is a two-time Edinburgh Festival Fringe sell-out (2018 and 2022) and has toured to London, Glasgow, Atlanta and Washington DC (2022). This 5-week run in NYC is timed to be in support of the High-Level Meeting on AMR (HLM AMR) during UNGA 2024. Go here for a blurb and here to book your tickets!
- [ONGOING] 17-20 Sep 2024 (Porto, Portugal; virtual attendance is possible): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 19 Sep 2024 (virtual, 10-11.30a CEST / 18.00–19.30 AEST): GARDP-sponsored webinar entitled “An introduction to antibiotic research and development (R&D).” Go here to register.
- 24 Sep 2024 (in person, 7.45-10a ET, New York City): Breakfast meeting entitled “Advancing Together: Securing the Global AMR Agenda by Harnessing the Collective Strength of Multi-Sector Partnerships”, sponsored by bioMèrieux, The Wellcome Trust, The American Society of Microbiology, and the Republic of Malawi. This occurs two days before the 26 Sep 2024 UNGA HLM on AMR. Go here to register.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 16 Oct 2024 (virtual and in-person, 10a-1p ET): FDA’s Rare Disease Innovation Hub, in collaboration with the Reagan-Udall Foundation will discuss how the recently announced Rare Disease Innovation Hub can engage and prioritize its work. This may seem somewhat remote, but could this have implications for rare infections? Hmm! Attend if you can! Go here for the meeting’s webpage.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- [NEW — short timeline on registration] 22-24 Oct 2024 (Belgrade, Serbia): Ecraid/ESCMID postgraduate course “Better methods for clinical studies in infectious diseases and clinical microbiology”. Go here to register by 29 Sep 2024.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.