Dear All,
I wrote in the 1 Dec 2024 newsletter about the excellent paper by Baraldi et al. that introduced us to the 6 possible meaning of “lack of access.” Since that newsletter, a bit of back-and-forth has produced a UNSLAP as a pronounceable acronym for the 6 meanings: (U)nregistered, (N)ever registered (or, not registered), (S)hort-term shortage, (L)ong-term shortage, (A)ffordability, and (P)hysical Presence of a Health Care Pfacility.
And now on the subject of (mostly) the idea of Short-term shortages, we have a joint WHO-GARDP report entitled “Policy and regulatory interventions to address antibiotic shortages in low and middle-income countries.” Here are the links you need:
- The website for the report and the report itself
- A press release about the report
- The joint WHO-GARDP SECURE project that seeks to expand access to essential antibiotics: https://www.secureantibiotics.org/about
Noting the focus of the report on shortages in LMICs (Low- and Middle-Income Countries), I was struck by one of the opening comments in the paper:
- “Antibiotic shortages in LMIC are often viewed as less urgent than in HIC, and only a few of the opinion leaders in LMIC who were interviewed recognized them as a major concern.
- “This is due to gaps in available treatment being masked by a general higher frequency of stock-outs, and potentially, by the circulation of substandard or falsified medicines.
- “…
- “As the health-care landscape evolves, antibiotic shortages are projected to become a larger concern in LMIC.
- “Once effective enforcement mechanisms are in place, the market for substandard, unregistered and falsified products will be removed, and, with more information on actual demand, gaps in supply will become more obvious.”
I had never thought about falsified products as obscuring real shortages … but it’s obvious once pointed out!
After giving us a thorough tour of causes of (mostly) acute shortages, the heart of the paper is this summary of high-level actions that would make a difference:
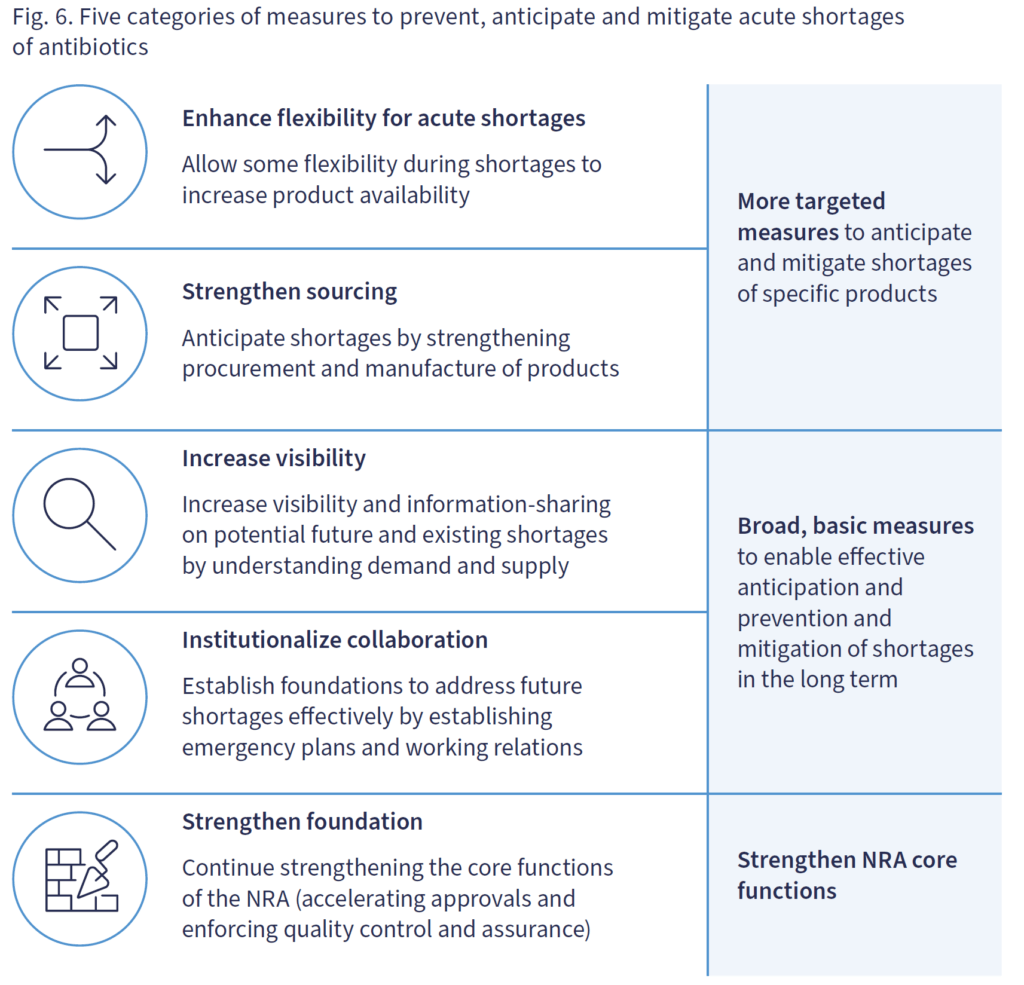
Once you know that NRA = National Regulatory Agency, the core ideas are a logical whole: adapt in ultra-short-term, coordinate/communicate for the longer term, and ensure that regulatory processes are adequate to keep up with it all.
And these ideas are (no surprise) consistent with the themes of SECURE, the joint WHO-GARDP project that “seeks to accelerate access to a portfolio of essential antibiotics, including generic antibiotics that are in short supply or not widely available, as well as newly approved Reserve antibiotics for multi-drug resistant bacterial infections.”
And finally, I’m told there will be a webinar in January on the report — I’ll post those details when I have them.
Nicely done, Team WHO and Team GARDP! All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- NIAID’s RFA-AI-24-069 is a notice of funding opportunity (NOFO) soliciting applications to establish Centers for Accelerating Phage Therapy to Combat ESKAPE Pathogens (CAPT-CEP). The CAPT-CEPs will focus on developing preclinical assays, tools, and models for robust phage therapy research and development (R&D) and advancing phage clinical research. Applications are due by 28 Jan 2025; letters of intent must be received 30 days prior to the due date. See also the 23 Oct 2024 newsletter about the RFA for a discussion of issues with developing phage-based products.
- The 2026 NIAID DMID Omnibus Broad Agency Announcement (HHS-NIH-NIAID-BAA2025-1) seeks applications in its Research Area 001 for (i) therapeutics for bacterial and fungal infections, (ii) vaccines for bacterial infections, and in vitro diagnostics for fungal pathogens. Applications are due by 21 Feb 2025. See also the 26 Nov 2024 newsletter discussing the BAA.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X has open calls at intervals that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- (no date as yet) 2025 ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to see details of the outstanding 2024 meeting!
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society of America. Details pending; go here for the general meeting website.
Upcoming meetings of interest to the AMR community:
- 23 Jan 2025 (virtual, 1700-1830 CET / 1100-1230 EST): GARDP REVIVE webinar entitled “The importance of chemical synthesis for antimicrobial R&D.” Go here to register.
- 28-29 Jan 2025 (online and in-person, Washington, DC): PACCARB (US Presidential Advisory Council on Combatting Antimicrobial Resistant Bacteria): This particular meeting of PACCARB is unusually important as it will seek (i) public input into NAP for CARB 2025-2030 and (ii) work to sustain the momentum regarding the commitments at the High-Level Meeting on AMR at the 2024 UN General Assembly (UNGA HLM AMR). Go to http://hhs.gov/paccarb for details and to register.
- 4-5 Feb 2025 (online, 1-5p GMT timing on both days): Antimicrobial Chemotherapy Conference by GARDP and BSAC in collaboration with CEPID-ARIES and Fiocruz. Now in its 6th year, the free program offers a good review of antimicrobial R&D, ranging from drug discovery to preclinical and clinical activities. Go here to register; the abstract deadline is 15 Nov 2024.
- [NEW] 5 Feb 2025 (The Spine, Liverpool): BioInfect 2025, Bionow’s annual north of England meeting for AMR researchers and industry professionals. The agenda includes a keynote lecture from Erin Duffy, Chief of Research & Development, CARB-X. Go here to register.
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. See list of Top Recurring meetings, above.
- 27 Feb 2025 (virtual, 1700-1830 CET / 1100-1230 EST): GARDP REVIVE webinar entitled “In vitro and in vivo correlations for prediction of human pharmacokinetics and dose of antimicrobials.” Go here to register.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025. See list of Top Recurring meetings, above.
- 30 June-1 July 2025 (virtual and in Washington, DC): BID2025: BARDA Industry Days — Enhancing Health Security With a Sustainable Future. BID provides the opportunity to discuss U.S. government medical countermeasure (MCM) priorities, provide the private sector an informal opportunity to interact with BARDA and ASPR teams, and identify potential areas of collaboration in the field of MCM research and development. Go here for details.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025. See list of Top Recurring meetings, above.
- 11-19 Oct 2025 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2025 will be the 9th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications should open ~March 2025 — go here for more details.
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.