Dear All,
WHO have just (2 Oct 2025) released a pair of reports on therapeutics and diagnostics for bacterial pathogens, with a focus on how these advance our tools for priority bacterial pathogens. Here are the links you need:
- WHO 2025 update on the preclinical and clinical antibacterial pipeline
- Title: “Analysis of antibacterial agents in clinical and preclinical development: overview and analysis 2025”
- Data cut-off: 15 Feb 2025
- Links: Webpage about the report, the report itself
- [9 Nov 2025 update]: There is now an interactive dashboard version that allows interrogation of both the clinical pipeline and the preclinical pipeline. In addition, an .xlsx version of the data is also available for download.
- WHO 2025 update on available and pipeline antibacterial diagnostics
- Title: “Landscape analysis of commercially available and pipeline in vitro diagnostics for bacterial priority pathogens”
- Data cut-off: 15 Dec 2024
- Links: Webpage about the report, the report itself
- [9 Nov 2025 update]: There is now an interactive dashboard that allows you to explore the data.
- Reference:
- High-level webpage regarding both reports
- The AMR.Solutions summary of the various bacterial priority pathogen lists (PPLs)
As always, there’s a lot to digest … but you can start with this topline summary:
- Clinical antibacterial pipeline
- The pipeline is shrinking and fragile: WHO identified 90 agents (down from 97 in 2023) of which 50 are traditional antibiotics and 40 are non-traditional approaches.
- Gaps remain notable for oral therapies for outpatient use as well as in the area of pediatric formulations
- Innovation remains limited: only 15 agents are considered innovative, but only 5 target WHO critical priority pathogens.
- Preclinical antibacterial pipeline
- 232 programs are being advanced by 148 groups, over 90% of which are led by small and micro tech companies, again underlining the system’s volatility.
- The focus remains heavily on Gram-negative pathogens.
- Diagnostic tools: Gaps are notable, especially in low-resource settings:
- Few point-of-care tools for primary and secondary care
- No multiplex platforms for level II labs to detect bloodstream infections directly from whole blood (Level I = Primary care setting; Level II = District setting; Level III = Regional; Level IV = National)
- Access to biomarker tests to distinguish bacterial from viral infections remains limited
The graphical view of the pipeline is instructive:
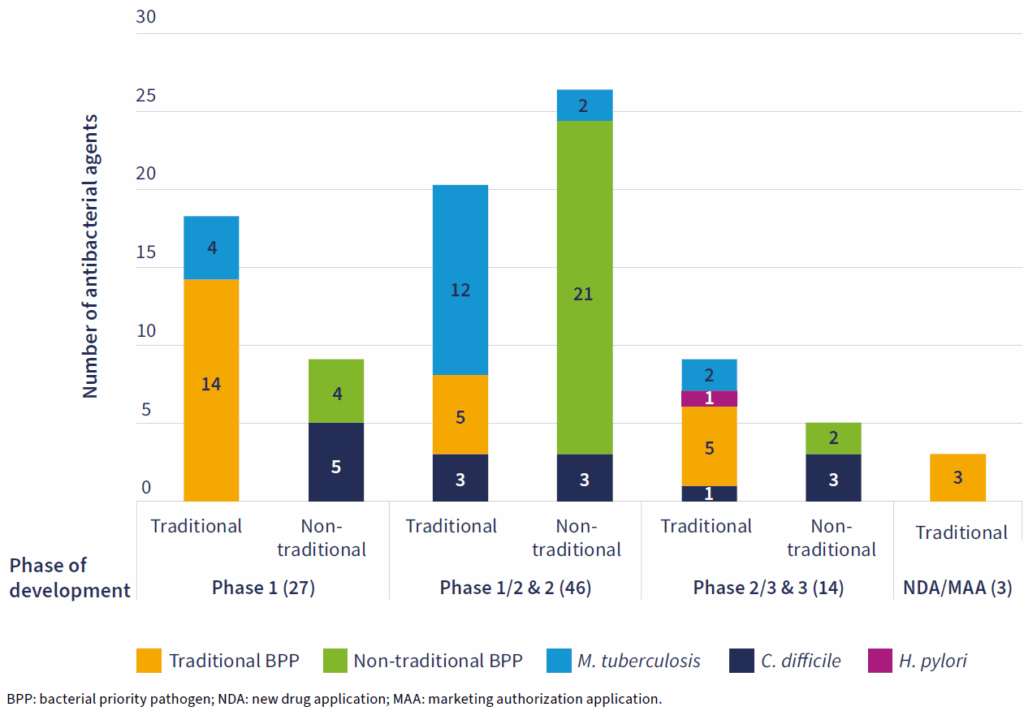
Singe-digits to low double-digits throughout! Ugh! When compared with the 1,000s of products in clinical development for cancer, this list is indeed disturbingly small!
As the thing that will really make a difference for therapeutics, my fingers (and toes) remain crossed for evidence of continued “Progress (at least some!) towards a global pull incentive” discussed in the 16 Sep 2025 newsletter. I was especially encouraged during the recent GAMRIC 2025 meeting about progress in Europe where TEVs do seem to be advancing (TEV = Transferrable Exclusivity Voucher; see 20 Jun 2025 newsletter, “Pull incentives in Europe: Next legislative steps”).
Solutions for diagnostics remain more elusive at present. But we did hear at GAMRIC 2025 that the idea of extending the STEDI framework to STRIDES (adding the letters (R)esearch value and (S)urveillance value) seems to hold promise as the basis of a path forward (18 June 2025 newsletter, “Extending STEDI to diagnostics: STRIDES”). More to follow on this, I hope!
Onward! All best wishes, –jr
PS: On this theme, note also the 13 Oct 2025 (1300-1415 CEST, virtual) WHO webinar launching their 2025 WHO Global Antibiotic Resistance Surveillance Report. Go here to register!
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers, the small meeting format of BEAM’s AMR Conference (March) and GAMRIC (September-October; formerly, the ESCMID-ASM conference series) creates excellent global networking. IDWeek (October) and ECCMID (April) are much larger meetings but also provide opportunities for networking with a substantial, focused audience via their Pipeline sessions. Hope to see you there!
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society of America. Go here to register. For those who would like a substantial opportunity to present a product to a large audience (see also adjacent note about ESCMID), note the call for applications to present at an IDWeek Pipeline Session; go here to submit an application for your compound or diagnostic.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference. Sponsored by the BEAM Alliance, the 9th AMR Conference has just concluded and it’s again been an excellent meeting! Please mark your calendar for next year. You can’t register yet, but details will appear here!
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. You can’t register yet, but you can go here for details on the outstanding 2025 meeting. The abstract submission window for 2026 will run 15 October to 26 Nov 2025. For those who would like a substantial opportunity to present a product to a large audience (see also adjacent note about IDWeek), I know that the meeting schedule will again include Pipeline Monday; go here to see details from 2025.
- [Next year’s date and location announced] 22-24 Sep 2026 (Lisbon, Portugal): The 2nd GAMRIC, the Global AMR Innovators Conference (London, UK). Formerly the ESCMID-ASM Joint Conference on Drug Development for AMR, 2026 will be the 11th year for this series under the joint sponsorship of CARB-X, ESCMID, BEAM Alliance, GARDP, LifeArc, Boston University, and AMR.Solutions. The ongoing series employs the successful format of prior meetings with a single-track meeting and substantial networking time. The 2025 meeting was a sell-out success — be sure to mark your calendar for 2026!
Upcoming meetings of interest to the AMR community:
- [NEW] 13 Oct 2025 (1300-1415 CEST, virtual): WHO webinar launching their 2025 WHO Global Antibiotic Resistance Surveillance Report
- 9-13 Nov 2025 (Portland, OR, USA): ASM Conference on Biofilms. Go here for details and to register.
- 18-24 Nov 2025 (global, multiple locations): World Antibiotic Awareness Week (WAAW) is convened annually on 18-24 Nov by WHO with national events (e.g., CDC’s US Antibiotic Awareness Week (USAAW); ECDC’s 18 Nov European Antibiotic Awareness Day) occurring around the globe. Details will follow as events become visible.
- 20 Nov 2025 (Washington, DC, 9a-5p ET): BARDA Innovation Symposium. BARDA will be showcasing early-stage health security innovations supported by BARDA, including through DRIVe, BARDA Accelerator Network, BARDA Ventures, Blue Knight, and CARB-X. The symposium (see also the 20 Sep 2025 newsletter: “20 Nov 2025: BARDA Innovation Symposium”) convenes “a diverse network of early stage companies, government agencies, non dilutive funders, investors, and strategic partners all interested in developing the next generation of medical countermeasures (MCMs).” BARDA has consistently been a very creative funder seeking very diverse types of products … could this be you? Go here for details and to register.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025. See list of Top Recurring meetings, above.
- 29-31 Oct 2025 (Bengalaru, India): ASM Global Research Symposium on the One Health Approach to Antimicrobial Resistance (AMR), hosted in partnership with the Centre for Infectious Disease Research (CIDR) at the Indian Institute of Science (IISc). Go here for details and to register.
- 28-30 Jan 2026 (Las Vegas, NV, USA): IDSA and ASM have announced a new US-based meeting series entitled IAMRI (Interdisciplinary Meeting on Antimicrobial Resistance and Innovation) and described as a “forum for collaboration and exploration around the latest advances in antimicrobial drug discovery and development.” Go here for more details, to register, and to submit an abstract (deadline for abstracts is 1 Oct 2025).
- 4-5 Feb 2026 (virtual, 8a-noon GMT on both days): Antimicrobial Chemotherapy Conference 2026, sponsored by BSAC and GARDP. Registration here: acc-conference.com. Abstracts are welcomed and can be submitted here; abstract deadline is Friday, 14 November 2025, 17:00 GMT.
- 18-20 Feb 2026 (Sydney, Australia, in person): The “AMR 2026 Summit”, hosted by the Fleming Initiative and Australia’s Science Agency, CSIRO. This event (website) will spotlight evidence-informed One Health approaches, practical solutions to implementation barriers, and strategies for public engagement, education, and advocacy. Space is limited, so (and sort of like applying to attend a Gordon Conference), please register your interest to attend here.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference sponsored by the BEAM Alliance. See list of Top Recurring meetings, above.
- 8-13 Mar 2026 (Renaissance Tuscany Il Ciocco, Italy): 2026 Gordon Research Conference (GRC) entitled “Antibacterials of Tomorrow to Combat the Global Threat of Antimicrobial Resistance.” A Gordon Research Seminar (GRS) will be held the weekend before (7-8 Mar) for young doctoral and post-doctoral researchers. Space for the GRS and the GRC is limited; for details and to apply, go here for the GRC and here for the GRS.
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 4-8 June 2026 (Washington, DC): ASM Microbe, the annual meeting of the American Society for Microbiology. The meeting format is evolving and next year will combine 3 meetings (ASM Health, ASM Applied and Environmental Microbiology, and ASM Mechanism Discovery) into one event. Go here for details.
- 22-24 Sep 2026 GAMRIC (Lisbon, Portugal), the Global AMR Innovators Conference (London, UK; formerly the ESCMID-ASM Joint Conference on Drug Development for AMR). See list of Top Recurring meetings, above..
- [placeholder] ??-?? Oct 2026 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2026 will be the 10th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Dates for next year will not be posted for some time, but go here for more details and put a reminder in your calendar to check back in the Spring if you are interested.
Self-paced courses, online training materials, and other reference materials:
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.
- GARDP’s REVIVE website provides an encyclopedia covering a range of R&D terms, recordings of prior GARDP webinars, a variety of viewpoint articles, and more! Check it out!
- GARDP’s https://antibioticdb.com/ is an open-access database of antibacterial agents.
- The CARB-X website provides a range of recordings from its webinars, bootcamps, and more. A bit of browsing would be time well spent!
- British Society for Antimicrobial Chemotherapy offers an eLearning section: Education – The British Society for Antimicrobial Chemotherapy.
- NNF (Novo Nordisk Foundation) have announced their “Challenge Programme 2026 – Unravelling the Pathways of Human Invasive Fungal Diseases. The call seeks applications from EU-centered consortia (global partners are possible) for research in 4 areas: (i) fungal virulence factors, (ii) host-pathogen interactions, (iii) mechanisms of anti-fungal resistance, and (iv) fungal disease markers. Applications are due by 8 Oct 2025. Go here for details.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X will have two calls during 2025 that span two areas: (i) Small molecules for Gram-negatives (the focus is on Pseudomonas aeruginosa) and (ii) Diagnostics for typhoid (the focus is diagnosis of acute infections in 60 minutes or less). See this 26 Feb 2025 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. The first cycle is now closed (it ran16-30 April 2025); the 2nd round will be open 1-12 Dec 2025.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Antimicrobial Resistance Research and Innovation in Australia is an actively updated summary that covers Australia’s AMR research and patent landscape. It is provided via collaboration between The Lens (an ambitious project seeking to discover, analyse, and map global innovation knowledge) and CSIRO (Commonwealth Scientific and Industrial Research Organisation, an Australian Government agency responsible for scientific research). Lots to explore here!
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).