Dear All,
On 26 Nov 2026, the Antimicrobial Resistance Surveillance & Research Initiative (AMRSRN) of the Indian Council on Medical Research (ICMR) released its report on AMR across India for 2024. The report is the eighth since AMRSRN started publishing such reports in 2017; the report for 2024 is here and links to all the prior reports are here. The 2024 report is based on data from almost 100,000 isolates from > 20 centers across India:
It’s quite a survey! And as you might guess from data on that number of isolates, the report is very dense. The best way to summarize its key message is by considering several of the figures where the authors show evolution of resistance over time.
Let’s start with Escherichia coli and Klebsiella pneumoniae:

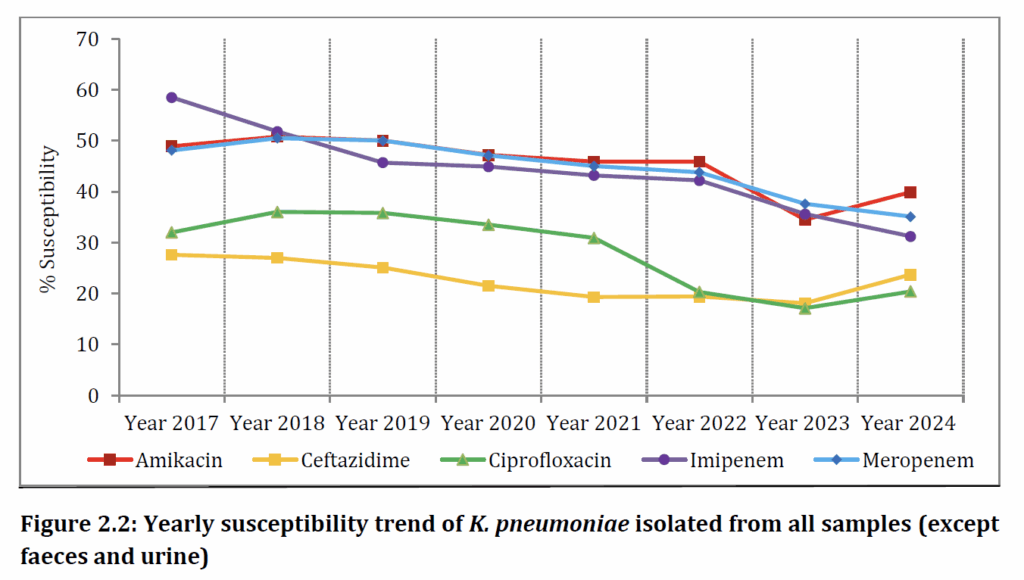
The readily evident and very disturbing trend is for steady falls in susceptibility across these standard drugs. Note that the survey does not include the newer agents (e.g., the newer beta-lactamase inhibitors), but you certainly get the picture regarding how resistance to any agent can steadily build.
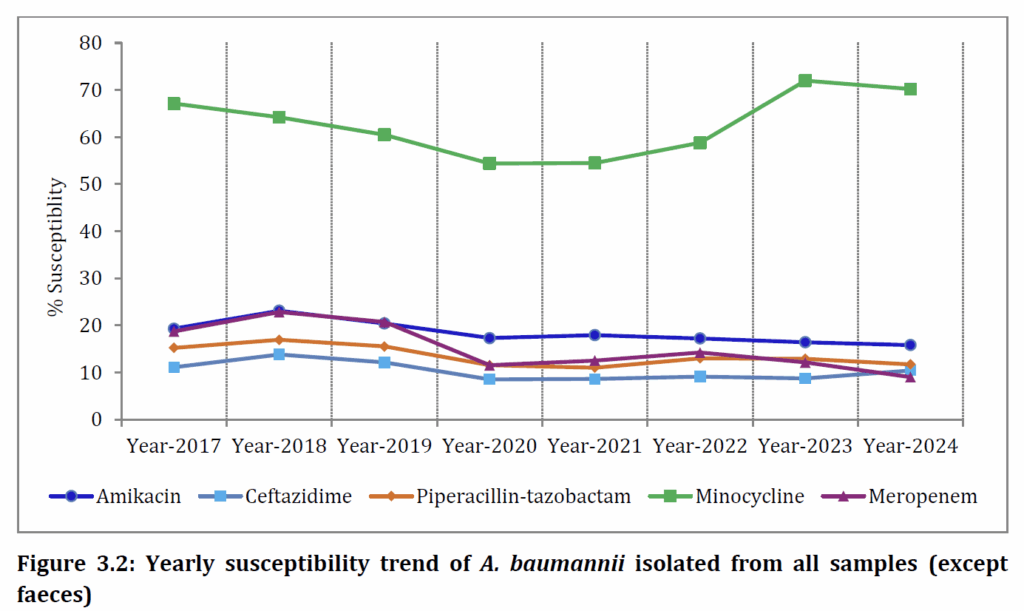
You also won’t be surprised at the picture for Acinetobacter baumannii where there is at least some susceptibility to minocycline (but still < 80%) and almost complete resistance to everything else. As noted above, the newer agents covering this pathogen are not (yet) in their survey.
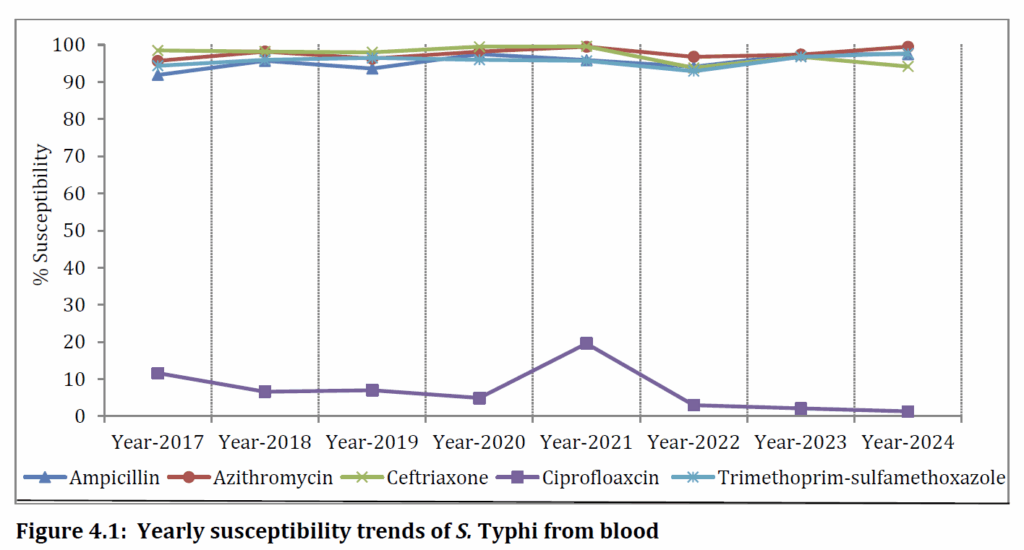
One bright spot was found in the data on Salmonella typhi (the causative agent for typhoid fever where ciprofloxacin resistance is nearly universal but good susceptibility is maintained thus far to ceftriaxone (98%), azithromycin (99.5%) trimethoprim-sulfamethoxazole (97.7%):
Deep sigh … and overall a worrisome picture! Our colleagues at ICMR are to be thanked for gathering and sharing these data!
But again a deep sigh. The good news is that newer agents are now available and hence the picture is not quite as bad as these graphs would suggest (provided you live in a territory that has these newer agents and that’s definitely not a given as we discussed in the 7 Apr 2025 newsletter entitled “UNSLAP: You reach for the antibiotic … and it’s not there!”).
But, the bad news is that resistance can and will emerge even to those new agents. Thus the title for this newsletter is “A possible global future unless we act now!” With only ~100 antibacterial agents in clinical development (see the 4 Oct 2025 newsletter discussing the most recent WHO report and also the other summaries at https://amr.solutions/pathogens-and-pipelines/), our buffer against the inevitable rise of resistance is very, very thin.
So, I am delighted to see efforts to drive R&D forward: the new CARB-X call, the research concepts from NIAID/DMID, and the initial call from the OHAMR initiative are all very welcome! But, the lesson of the 9 Apr 2024 newsletter is “48,015 → 0: Antibacterial discovery is hard. Really, really hard” … and in parallel we urgently need to build and maintain good financial Push and Pull mechanisms for both supporting R&D and for making agents available after initial approval. In particular right now, I have my fingers (and toes) crossed for the the eagerly watched work in the EU on Transferable Exclusivity Vouchers.
It’s a marathon, not a sprint … we need to keep working steadily to create and make available new options … none of us are safe until we are all safe! With thanks again to our colleagues at ICMR for sharing these data and with best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers, the small meeting format of BEAM’s AMR Conference (March) and GAMRIC (September-October; formerly, the ESCMID-ASM conference series) creates excellent global networking. IDWeek (October) and ECCMID (April) are much larger meetings but also provide opportunities for networking with a substantial, focused audience via their Pipeline sessions. Hope to see you there!
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference. Sponsored by the BEAM Alliance, the 9th AMR Conference was an excellent meeting! A draft program has been posted and registration is now open. Please plan to attend!
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. You can go here to register and view the preliminary program; the abstract submission window for 2026 will run 15 October to 26 Nov 2025. For those who would like a substantial opportunity to present a product to a large audience (see also adjacent note about IDWeek), I know that the meeting schedule will again include Pipeline Monday.
- 22-24 Sep 2026 (Lisbon, Portugal): The 2nd GAMRIC, the Global AMR Innovators Conference (London, UK). Formerly the ESCMID-ASM Joint Conference on Drug Development for AMR, 2026 will be the 11th year for this series that is now under the joint sponsorship of CARB-X, ESCMID, BEAM Alliance, GARDP, LifeArc, Boston University, and AMR.Solutions.
- The ongoing series employs the successful format of prior meetings with a single-track meeting and substantial networking time. The 2025 meeting was a sell-out success and the video from the sessions is now available here.
- [Don’t miss this: 16 Dec 2025 update webinar] The GAMRIC program committee is hosting an update webinar on 16 Dec 2025 (8-9a ET, 2-3 CET) that will include a review of the 2025 content and brainstorming on content for 2026. Go here to register! Don’t miss it!
- 21-24 Oct 2026 (Washington, DC, USA): IDWeek 2026, the annual meeting of the Infectious Diseases Society of America. Details are not yet available but I would expect the program to continue to provide a substantial opportunity to present a product to a large audience (see also adjacent note about ESCMID) as well as opportunities to present at an IDWeek Pipeline Session.
Upcoming meetings of interest to the AMR community:
- [NEW] 15 Dec 2025 (virtual, 13.00-14.30 CET): WHO sponsored webinar entitled “AMU Surveillance Data for Action” and will focus on using AMU surveillance data to inform policy making, stewardship, and access strategies. Go here to register. This webinar is part of a WHO series support implementation of national action plans — go here to see past webinars.
- [NEW — A virtual training course!] 21 Jan to 15 April 2026 (every Wednesday, 11a-12.30p MST / 1p-2.30p EST / 6-7.30p GMT / 7-8.30p CET): CAN-AMR-Net (Canadian Antimicrobial Resistance Network) is running a 12-lecture in-depth training course entitled “Antibiotic Drug Discovery: From AI-Enabled Discovery to Successful Commercialization Comprehensive Training.”
- The course will span the entirety of antibiotic development from early discovery to clinical, regulatory, and marketing stages. Go here for more details on the planned lectures and to register.
- To best enable interaction, seating is limited – please register promptly if you are interested. The course fee ranges from $100 to $500 CAD based on geography and background.
- CAN-AMR-Net is a Health Research Training Platform (HRTP) funded by the Canadian Institutes of Health Research (CIHR) from 2024 to 2030 and this course is part of their goal to train the next generation of researchers, practitioners, and leaders in the application of transdisciplinary methodologies and communication skills across One Health sectors to tackle the problem of AMR. For questions or suggestions on the course contents, contact Sameeh Salama at: ssalama@fedorapharma.com
- 28-30 Jan 2026 (Las Vegas, NV, USA): IDSA and ASM have announced a new US-based meeting series entitled IMARI (Interdisciplinary Meeting on Antimicrobial Resistance and Innovation) that is described as a “forum for collaboration and exploration around the latest advances in antimicrobial drug discovery and development.” Go here for to register. The window for general abstracts is closed, but a call for abstracts presenting new agents is now open through 1 Dec 2025.
- 4-5 Feb 2026 (virtual, 8a-noon GMT on both days): Antimicrobial Chemotherapy Conference 2026, sponsored by BSAC and GARDP. Registration here: acc-conference.com. Abstracts are welcomed and can be submitted here; abstract deadline is Friday, 14 November 2025, 17:00 GMT.
- 5 Feb 2026 (in person, Alderley Park, UK): BioInfect, the annual AMR-focused networking conference delivered by BioNow. Go here for details and to register.
- [NEW and IMPORTANT] 5-6 Feb 2026 (virtual or in person [FDA White Oak campus, 1.30-5p ET on 5 Feb; 8.30-4p ET on 6 Feb): FDA hosted public workshop #10 in their series entitled Advancing the Development of Pediatric Therapeutics (ADEPT). The goal is to discuss challenges seen in neonatal and rare disease product development and to identify ways to leverage rare disease product development tools and regulatory frameworks. Go here for more details and here to register. The challenges of pediatric development run deep … and we need to find ways to protect children by including them in research rather than excluding them. On this theme, you might enjoy reviewing the related 7 Apr 2021 newsletter (“Developing antibiotics for children: There are no easy answers”) and the 27 May 2022 newsletter (“Antibacterial guidance (including pediatrics): Parallel EMA+FDA updates”).
- 18-20 Feb 2026 (Sydney, Australia, in person): The “AMR 2026 Summit”, hosted by the Fleming Initiative and Australia’s Science Agency, CSIRO. This event (website) will spotlight evidence-informed One Health approaches, practical solutions to implementation barriers, and strategies for public engagement, education, and advocacy. Space is limited, so (and sort of like applying to attend a Gordon Conference), please register your interest to attend here.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference sponsored by the BEAM Alliance. See list of Top Recurring meetings, above.
- 8-13 Mar 2026 (Renaissance Tuscany Il Ciocco, Italy): 2026 Gordon Research Conference (GRC) entitled “Antibacterials of Tomorrow to Combat the Global Threat of Antimicrobial Resistance.” A Gordon Research Seminar (GRS) will be held the weekend before (7-8 Mar) for young doctoral and post-doctoral researchers. Space for the GRS and the GRC is limited; for details and to apply, go here for the GRC and here for the GRS.
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 4-8 June 2026 (Washington, DC): ASM Microbe, the annual meeting of the American Society for Microbiology. The meeting format is evolving and next year will combine 3 meetings (ASM Health, ASM Applied and Environmental Microbiology, and ASM Mechanism Discovery) into one event. Go here for details.
- 22-24 Sep 2026 GAMRIC (Lisbon, Portugal), the Global AMR Innovators Conference (London, UK; formerly the ESCMID-ASM Joint Conference on Drug Development for AMR). See list of Top Recurring meetings, above..
- 10-18 Oct 2026 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2026 will be the 10th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Registration for 2026 will not open for some time; go here for more details and put a reminder in your calendar to check back in the Spring if you are interested.
- 21-24 Oct 2026 (Washington, DC, USA): IDWeek 2026. See list of Top Recurring meetings, above.
Self-paced courses, online training materials, and other reference materials:
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.
- GARDP’s REVIVE website provides an encyclopedia covering a range of R&D terms, recordings of prior GARDP webinars, a variety of viewpoint articles, and more! Check it out!
- GARDP’s https://antibioticdb.com/ is an open-access database of antibacterial agents.
- The CARB-X website provides a range of recordings from its webinars, bootcamps, and more. A bit of browsing would be time well spent!
- British Society for Antimicrobial Chemotherapy offers an eLearning section: Education – The British Society for Antimicrobial Chemotherapy.
- [CALL IS NOW OPEN] CARB-X has now opened its 2nd call for 2025. The call is focused on (i) Small molecules for Gram-negatives (the focus is on Pseudomonas aeruginosa) and (ii) Diagnostics for typhoid (the focus is diagnosis of acute infections in 60 minutes or less). See this 26 Feb 2025 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. This 2nd round is open 1-12 Dec 2025. Informational webinars are being held on Thu 4 Dec at 19:00 ET and Fri 5 Dec at 9:00 ET.
- EU OHAMR (One Health AMR) has opened its first call with a request for consortia to propose projects on (i) combination therapies, (ii) ways to improve adherence to protocols, and (iii) ways to assess/inform regarding the impact of antimicrobials used in veterinary medicine and food agriculture. The window for pre-proposals runs 18 Nov 2025 to 2 Feb 2026. See also the 22 Oct 2025 newsletter about the call.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Antimicrobial Resistance Research and Innovation in Australia is an actively updated summary that covers Australia’s AMR research and patent landscape. It is provided via collaboration between The Lens (an ambitious project seeking to discover, analyse, and map global innovation knowledge) and CSIRO (Commonwealth Scientific and Industrial Research Organisation, an Australian Government agency responsible for scientific research). Lots to explore here!
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).