Dear All (wonkish … but worth it … get ready to follow the links!),
Greetings in the new year! As a marvelous way to end the last year, the US FDA announced on 12 Dec 2025 its approval of two new oral therapies for gonorrhea, thus addressing the priority pathogen lists of both WHO (High priority) and CDC (Urgent) due to the rise in resistant infections. Achieving this has been a huge lift for reasons we’ll discuss below. To get you started, here are the main links you need:
- The US labels for the two new drugs
- Gepotidacin (Blujepa, developed by GSK)
- The pivotal Phase 3 study supporting approval is now published as Ross et al., The Lancet, https://doi.org/10.1016/S0140-6736(25)00628-2.
- Zoliflodacin (Nuzolvence, developed by Innoviva Specialty Therapeutics and GARDP)
- The pivotal Phase 3 study supporting approval is now published as Luckey et al., The Lancet, https://doi.org/10.1016/S0140-6736(25)01953-1
- Background materials
- Hook EW et al. Development of New Antimicrobials for Urogenital Gonorrhea Therapy: Clinical Trial Design Considerations. Clinical Infectious Diseases. 2020;70(7):1495–500, https://doi.org/10.1093/cid/ciz899. This is an excellent review of the challenges of developing new therapies for gonorrhea.
- 23 April 2021 FDA workshop on developing drugs for gonorrhea. The superb materials from this workshop are readily available online at that link. You would find value in reviewing them all, but to begin please look at these four presentations:
- Ann Jerse: Animal models for pre-clinical testing of antibiotics against gonorrhea: Established and new models under development
- Hiwot Hiruy (FDA): Development of Antibacterial Drugs for Uncomplicated Gonorrhea: A Regulatory Perspective
- Radu Botgros (EMA): Regulatory Perspectives on Development of Antibacterial Medicines for Gonorrhoea
- Sue Cammarata: Overview of Drug Development Considerations for Uncomplicated Urogenital Gonorrhea a Tale of Two Recent Trials
- 22 Feb 2026 addendum: A manuscript summarizing the workshop is now available as Hiruy H et al. Development Considerations of Antimicrobial Drugs for the Treatment of Gonorrhea. Clinical Infectious Diseases. 2026;81(6):1201–8; doi: 10.1093/cid/ciae386.
- Current guidance documents on developing drugs for uncomplicated gonorrhea
- FDA’s most current guidance is from 2015 … I would expect the materials above to be the basis for an update.
- EMA included gonorrhea in their 2022 general antibacterial guidance.
- See also the 27 Mar 2022 newsletter (“Antibacterial guidance (including pediatrics): Parallel EMA+FDA updates”) for comments and connections.
- Note that both drugs are approved for children down to age 12. It’s really good that the sponsors went ahead and included adolescents as the delays in access for children are SO frustrating! For more on pediatric development, see:
- 27 May 2022 newsletter: “Antibacterial guidance (including pediatrics): Parallel EMA+FDA updates”
- 7 Apr 2021 newsletter: “Developing antibiotics for children: There are no easy answers”
- Gepotidacin (Blujepa, developed by GSK)
So, what are the key messages from these materials? Well, let’s start with the usual points that (i) developing new antibiotics is really, really hard (4 April 2025 newsletter), (ii) the trials are slow and expensive (30 June 2020 newsletter summarizing FDA’s 40-year experience), (iii) the patients with greatest unmet need are hard to enroll, and (iv) the return on investment is generally negative.
And although it is not obvious given the reasonably high frequency of sexually transmitted infections, gonorrhea (hereinafter abbreviated as NG for Neisseria gonorrhea) poses several additional hurdles to developers. To get us started on these further issues, Sue Cammarata’s discussion of two drugs that failed in Phase 3 provides this summary:
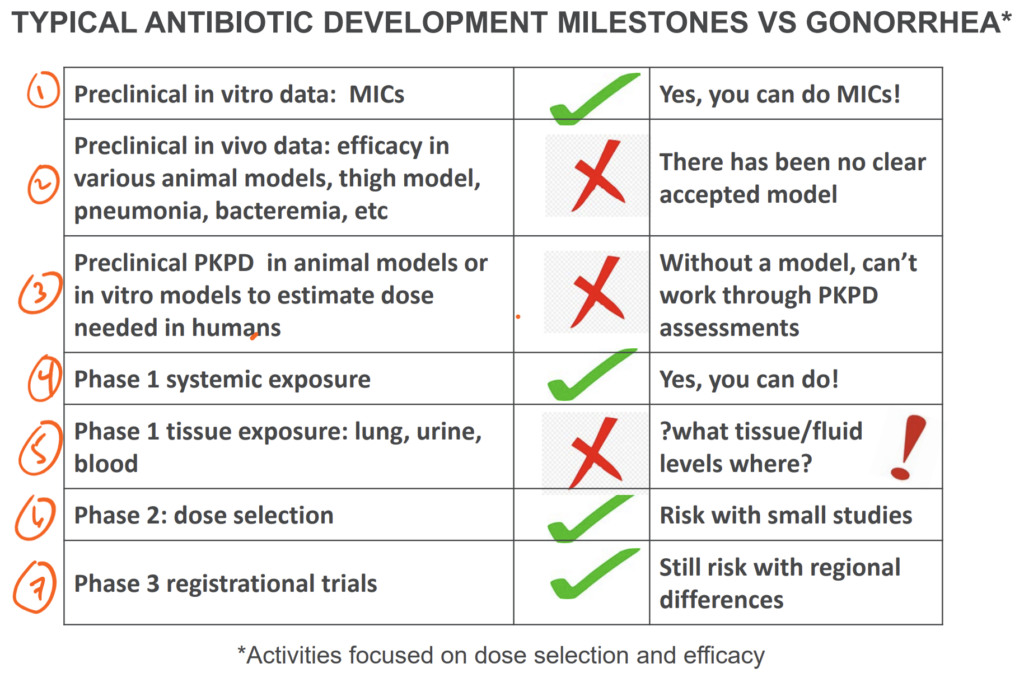
What are the challenges that led to the failure of those two drugs in Phase 3? With the aid of the comments from Hook 2020, let’s walk through the ideas in the slide above:
First (and just about the only bit of good news in this list of challenges), NG itself is readily tested in the laboratory. It can be cultured and MICs can be generated. But, it turns out that it also readily mutates to become resistant. Unemo and Shafer gave us a vivid graphical illustration of the problem in their 2014 paper (Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587–613, https://doi.org/10.1128/cmr.00010-14):
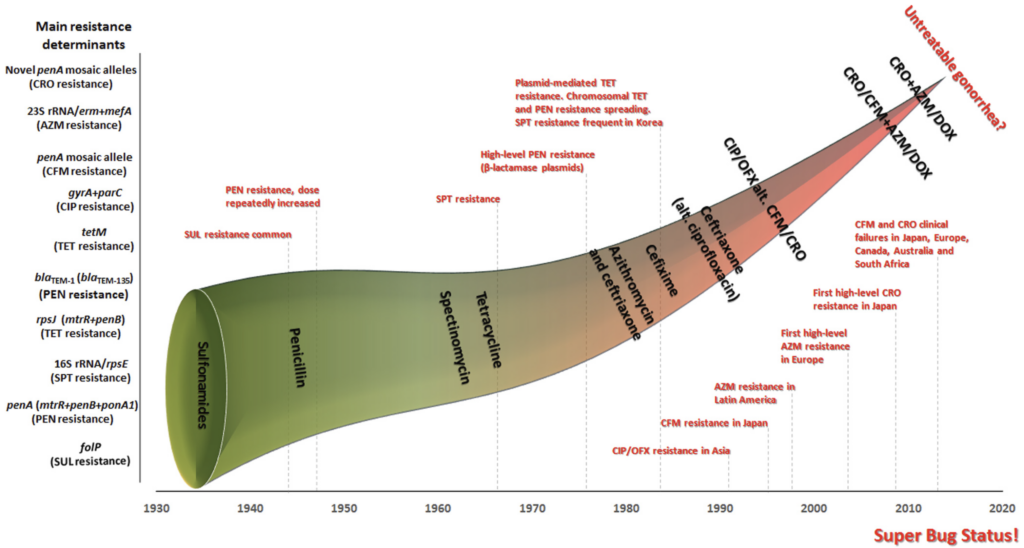
Note that the x-axis of the figure above is time (1930-2020) and the y-axis is the accumulation of resistance determinants. As the funnel of available drugs gets smaller and smaller, we have the ongoing evolution of highly resistant infections.
Second, preclinical animal models of NG have been hard to create because humans are the only natural hosts for NG. As summarized in the presentation by Ann Jerse, creating faithful mimics of human infection has only recently become possible. Third, those models do not fully encompass important body site differences (e.g., genital infection vs. upper reproductive tract infections). Collectively, these are challenges for reliable PK-PD predictions and hence for reliable dose selection.
Fourth (and fifth), Phase 1 studies are of course possible, but assessing PK at the diverse relevant body sites is problematic. GC is most often symptomatic in the male urethra where as women can be entirely asymptomatic. Infection can also be endometrial, in the ovarian ducts, or the pharynx … and all of these sites may have different PK properties.
Sixth and seventh, Phase 2 and Phase 3 studies encounter further issues that tend to reduce enrollment of women and children (and thus limit the quantity/quality of data available for these groups):
- The differences in presentation (symptomatic in men, [often] asymptomatic in women) mean that it’s hard to enroll enough women in trials.
- It can be harder to culture NG from women during certain points in the menstrual cycle.
- Women must also be screened for pregnancy and (generally) not enrolled (although the thinking on this is evolving towards a much more nuanced benefit-risk consideration — see the summary by Sewell et al. of an excellent 2021 Duke-Margolis workshop on this topic).
- Likewise, limitations on enrolling children generally mean that sexually active adolescents are excluded.
- For statistical reasons driven by the high cure rate of a good comparator in patients with susceptible infections (as well as the expectation of a 10% non-inferiority margin), the required studies (at least for Phase 3) tend to be relatively large.
Finally, and even though it doesn’t cause failure of trial programs, I’ll add an eighth challenge: return on investment (ROI). As you’ve probably realized by now, part of the reason that therapies for chronic diseases are well reimbursed as that the drugs are needed for extended periods (if not for life). In the case of gonorrhea, however, the focus on developing single-dose therapies really works against adequate ROI … it’s difficult enough with 2-week therapies … but cure via a single dose really takes this challenge to its logical limit.
—
Whew! My hat is off to the teams that brought these products to initial approval! Well done! And now comes the equally large challenge of making these agents available globally. It will be interesting to see how this evolves:
- GARDP have published a case study of their work to develop zoliflodacin in which they note that they seek to use sub-licensing agreements to make zoliflodacin available soon in Thailand and South Africa (two countries that participated in their Phase 3 study).
- Per a personal communication with the GSK development team, I can also say that GSK is currently exploring potential pathways to make gepotidacin available in other countries where there is a medical unmet while preserving gepotidacin’s long-term effectiveness with robust surveillance and strong antimicrobial stewardship. To this end, I would make the guess that GSK will be leveraging their 20+ years of collaborations on neglected tropical diseases (NTDs) in support of access to antimicrobials in general. As an example, GSK has a collaboration with AMREF Health Africa to build generalized frameworks supporting responsible and equitable antibiotic access (along with appropriate use) in regions with unmet need.
- The work planned by both groups is consistent with the ideas from the 2021 Stewardship and Access guidance (22 Mar 2021 newsletter) that was jointly developed by CARB-X, Wellcome, BARDA, GARDP, and others.
Looking to the long-term, having two new drugs is marvelous … but resistance will doubtless develop over time to both. We’ll need further agents and we need a vaccine! It’s been interesting to see data emerge (Abara 2025 JID, https://doi.org/10.1093/infdis/jiae383 is a good review) showing that the vaccine for Neisseria meningitidis provides at least some protection vs. Neisseria gonorrhea. This would seem to bode well for further work!
With thanks to the many, many colleagues who have worked for years to bring this drugs to this point and with best wishes for 2026, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers, the small meeting format of BEAM’s AMR Conference (March) and GAMRIC (September-October; formerly, the ESCMID-ASM conference series) creates excellent global networking. IDWeek (October) and ECCMID (April) are much larger meetings but also provide opportunities for networking with a substantial, focused audience via their Pipeline sessions. Hope to see you there!
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference. Sponsored by the BEAM Alliance, the 9th AMR Conference was an excellent meeting! A draft program has been posted and registration is now open. Please plan to attend!
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. You can go here to register and view the preliminary program; the abstract submission window for 2026 will run 15 October to 26 Nov 2025. For those who would like a substantial opportunity to present a product to a large audience (see also adjacent note about IDWeek), I know that the meeting schedule will again include Pipeline Monday.
- 22-24 Sep 2026 (Lisbon, Portugal): The 2nd GAMRIC, the Global AMR Innovators Conference (London, UK). Formerly the ESCMID-ASM (or ASM-ESCMID depending on location) Joint Conference on Drug Development for AMR, 2026 will be the 11th year for this series that is now under the joint sponsorship of CARB-X, ESCMID, BEAM Alliance, GARDP, LifeArc, Boston University, and AMR.Solutions. The ongoing series employs the successful format of prior meetings with a single-track meeting and substantial networking time. The 2025 meeting was a sell-out success! A written summary of the meeting is here and the video from the sessions is now available here. Registration will open March 2026; the abstract submission window will be 10-31 March 2026.
- 21-24 Oct 2026 (Washington, DC, USA): IDWeek 2026, the annual meeting of the Infectious Diseases Society of America. Details are not yet available but I would expect the program to continue to provide a substantial opportunity to present a product to a large audience (see also adjacent note about ESCMID) as well as opportunities to present at an IDWeek Pipeline Session.
Upcoming meetings of interest to the AMR community:
- [NEW] 21 Jan 2026 (virtual, 18.30-19.45 CET): The World Economic Forum’s annual meeting in Davos will include the broadcast of an Open Forum event entitled “The Fragile Future of Antibiotics.” Go here for most current details on the program; the link to the livestream should become visible at that same link. A recording will be available after the event.
- 21 Jan to 15 April 2026 (every Wednesday, 11a-12.30p MST / 1p-2.30p EST / 6-7.30p GMT / 7-8.30p CET): CAN-AMR-Net (Canadian Antimicrobial Resistance Network) is running a 12-lecture in-depth training course entitled “Antibiotic Drug Discovery: From AI-Enabled Discovery to Successful Commercialization Comprehensive Training.”
- The course will span the entirety of antibiotic development from early discovery to clinical, regulatory, and marketing stages. Go here for more details on the planned lectures and to register.
- To best enable interaction, seating is limited – please register promptly if you are interested. The course fee ranges from $100 to $500 CAD based on geography and background.
- CAN-AMR-Net is a Health Research Training Platform (HRTP) funded by the Canadian Institutes of Health Research (CIHR) from 2024 to 2030 and this course is part of their goal to train the next generation of researchers, practitioners, and leaders in the application of transdisciplinary methodologies and communication skills across One Health sectors to tackle the problem of AMR. For questions or suggestions on the course contents, contact Sameeh Salama at: ssalama@fedorapharma.com
- 28-30 Jan 2026 (Las Vegas, NV, USA): IDSA and ASM have announced a new US-based meeting series entitled IMARI (Interdisciplinary Meeting on Antimicrobial Resistance and Innovation) that is described as a “forum for collaboration and exploration around the latest advances in antimicrobial drug discovery and development.” Go here for details and to register.
- 4-5 Feb 2026 (virtual, 8a-noon GMT on both days): Antimicrobial Chemotherapy Conference 2026, sponsored by BSAC and GARDP. Registration here: acc-conference.com. Abstracts are welcomed and can be submitted here; abstract deadline is Friday, 14 November 2025, 17:00 GMT.
- 5 Feb 2026 (in person, Alderley Park, UK): BioInfect, the annual AMR-focused networking conference delivered by BioNow. Go here for details and to register.
- 5-6 Feb 2026 (virtual or in person [FDA White Oak campus, 1.30-5p ET on 5 Feb; 8.30-4p ET on 6 Feb): FDA hosted public workshop #10 in their series entitled Advancing the Development of Pediatric Therapeutics (ADEPT). The goal is to discuss challenges seen in neonatal and rare disease product development and to identify ways to leverage rare disease product development tools and regulatory frameworks. Go here for more details and here to register. The challenges of pediatric development run deep … and we need to find ways to protect children by including them in research rather than excluding them. On this theme, you might enjoy reviewing the related 7 Apr 2021 newsletter (“Developing antibiotics for children: There are no easy answers”) and the 27 May 2022 newsletter (“Antibacterial guidance (including pediatrics): Parallel EMA+FDA updates”).
- 18-20 Feb 2026 (Sydney, Australia, in person): The “AMR 2026 Summit”, hosted by the Fleming Initiative and Australia’s Science Agency, CSIRO. This event (website) will spotlight evidence-informed One Health approaches, practical solutions to implementation barriers, and strategies for public engagement, education, and advocacy. Space is limited, so (and sort of like applying to attend a Gordon Conference), please register your interest to attend here.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference sponsored by the BEAM Alliance. See list of Top Recurring meetings, above.
- 8-13 Mar 2026 (Renaissance Tuscany Il Ciocco, Italy): 2026 Gordon Research Conference (GRC) entitled “Antibacterials of Tomorrow to Combat the Global Threat of Antimicrobial Resistance.” A Gordon Research Seminar (GRS) will be held the weekend before (7-8 Mar) for young doctoral and post-doctoral researchers. Space for the GRS and the GRC is limited; for details and to apply, go here for the GRC and here for the GRS.
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 4-8 June 2026 (Washington, DC): ASM Microbe, the annual meeting of the American Society for Microbiology. The meeting format is evolving and next year will combine 3 meetings (ASM Health, ASM Applied and Environmental Microbiology, and ASM Mechanism Discovery) into one event. Go here for details.
- 22-24 Sep 2026 GAMRIC (Lisbon, Portugal), the Global AMR Innovators Conference (London, UK; formerly the ESCMID-ASM Joint Conference on Drug Development for AMR). See list of Top Recurring meetings, above..
- 10-18 Oct 2026 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2026 will be the 10th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Registration for 2026 will not open for some time; go here for more details and put a reminder in your calendar to check back in the Spring if you are interested.
- 21-24 Oct 2026 (Washington, DC, USA): IDWeek 2026. See list of Top Recurring meetings, above.
- [NEW] 10-13 November 2026 (Madrid, Spain): The International Society for Infectious Diseases (ISID) has announced its 21st International Congress on Infectious Diseases (ICID). Register and view the preliminary program here. Note as well that the organizers have an open call for topic proposals with a 20 Jan 2026 deadline.
Self-paced courses, online training materials, and other reference materials:
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.
- GARDP’s REVIVE website provides an encyclopedia covering a range of R&D terms, recordings of prior GARDP webinars, a variety of viewpoint articles, and more! Check it out!
- GARDP’s https://antibioticdb.com/ is an open-access database of antibacterial agents.
- The CARB-X website provides a range of recordings from its webinars, bootcamps, and more. A bit of browsing would be time well spent!
- British Society for Antimicrobial Chemotherapy offers an eLearning section: Education – The British Society for Antimicrobial Chemotherapy.
- EU OHAMR (One Health AMR) has opened its first call with a request for consortia to propose projects on (i) combination therapies, (ii) ways to improve adherence to protocols, and (iii) ways to assess/inform regarding the impact of antimicrobials used in veterinary medicine and food agriculture. The window for pre-proposals runs 18 Nov 2025 to 2 Feb 2026. See also the 22 Oct 2025 newsletter about the call.
- The Horizon Europe Work Programme 2026-2027 includes at least 3 calls of interest within its Cluster 1 — see the list below. The application window starts 10 Feb 2026 and closes on 16 Apr 2026. See also the 12 Dec 2025 newsletter about the call. Note as well that there calls for agents to prevent and/or treat viral infections.
- HORIZON-HLTH-2027-01-DISEASE-08: Development of innovative antimicrobials against pathogens resistant to antimicrobials
- HORIZON-HLTH-2027-02-IND-02: Portable point-of-care diagnostics
- HORIZON-HLTH-2026-01-DISEASE-03:Advancing research on the prevention, diagnosis, and management of post-infection long-term conditions.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- CARB-X had an open call (its 2nd call for 2025) that ran 1-12 Dec 2025. I am sure there will be calls in 2026 and I’ll announce them when they are published.
- These things aren’t sources of funds but would help you develop funding applications
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Antimicrobial Resistance Research and Innovation in Australia is an actively updated summary that covers Australia’s AMR research and patent landscape. It is provided via collaboration between The Lens (an ambitious project seeking to discover, analyse, and map global innovation knowledge) and CSIRO (Commonwealth Scientific and Industrial Research Organisation, an Australian Government agency responsible for scientific research). Lots to explore here!
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).