Bugs & Drugs
I found that I was losing the plot about what was where! Here’s my quick guide to (i) priority pathogens (just below), (ii) pipeline reviews, and (iii) antibiotic manufacturing standards.
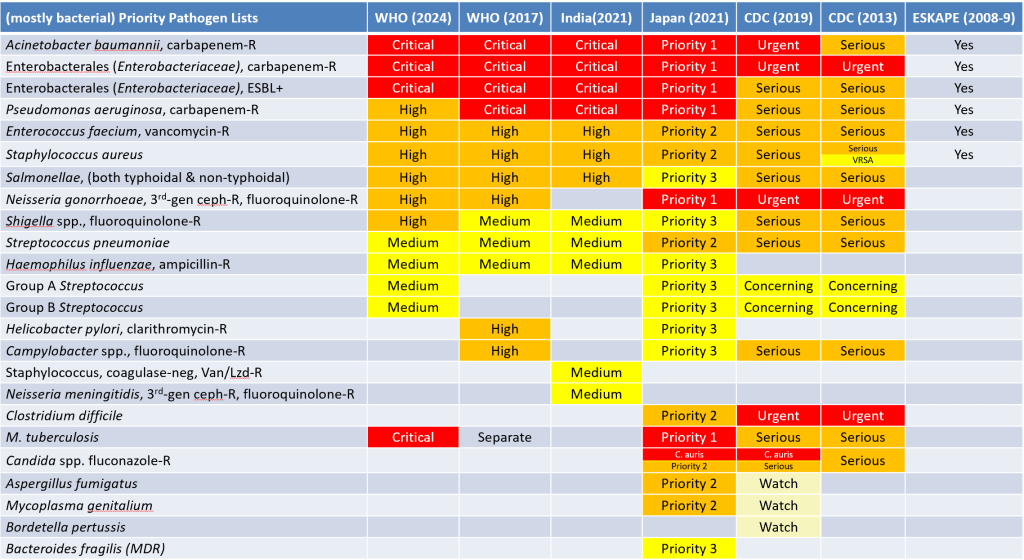
Note in particular that a comparative Summary of the priority pathogens lists is provided. For my summary of incentives to support this pipeline, see this webpage. Corrections and amends gratefully received.
Priority Pathogen / Threat Lists
- By my count, eight priority pathogen lists have been proposed / published to date; all are mainly bacterial unless otherwise noted:
- WHO 2024 PPL: Newsletter is here, report is here.
- WHO 2022 Fungal PPL: Newsletter is here, report is here.
- India 2021: Newsletter is here, report is here.
- Japan 2021: Summary in English; Extended details in Japanese
- CDC 2019: Newsletter is here, report itself is here.
- WHO 2017: Newsletter is here, report itself is here.
- CDC 2013: Report itself is here.
- ESKAPE list 2008-9: This is the grandfather/mother of them all! The original papers are here:
- (link) Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079-81.
- (link) Boucher HW et al. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009;48(1):1-12
To help you keep them organized, a comparative summary of the priority pathogen lists (including the 2022 WHO fungal PPL) as a PowerPoint deck is here (or here as a .pdf).
- Please also see this newsletter for a further discussion of the priority pathogen lists as well as the idea of first- vs. best-in-class.
Pipeline Reviews (therapeutic products for bacterial infections, unless otherwise noted)
- Reviews by WHO
- WHO 2024 antifungal preclinical and clinical pipeline review (data cut-off of 1 Sep 2024, published 1 April 2025): My newsletter is here and the WHO report (plus a great dashboard!) is here.
- WHO 2024 antifungal diagnostic pipeline review (data cut-off of 1 July 2024, published 1 April 2025): My newsletter is here and the WHO report is here.
- WHO 2023/24 preclinical and clinical pipeline review (data cut-off of 31 Dec 2023, published in 2024): My newsletter is here and WHO’s report is here. There is also a summary of the report (nice graphics!) with an editorial in Lancet Microbe.
- WHO 2022 review of preclinical and clinical vaccines (data cut-off not stated, appears to be from 2010 forward). Here are links to the report, a press release, and a newsletter about the report.
- WHO 2021/22 clinical pipeline review (data cut-off 30 June 2021, published in 2022): My newsletter is here and the review itself is here.
- WHO 2020 (data cut-off 1 Sep 2020): My newsletter is here (and includes a video chat with Peter Beyer, one of the key drivers of the report). The review itself is here.
- WHO 2019: My newsletter is here, commentary by WHO staff is here, the pre-clinical pipeline review is here and is supported by a data-mining interface here; the clinical pipeline review is here.
- WHO 2017: Newsletter is here, report is here, and manuscript about the review is here.
- Reviews by the Pew Trusts (all updated as of March 2021; see also this newsletter)
- Independent pipeline reviews
- Preclinical pipeline review: Theuretzbacher, U., Jumde, R.P., Hennessy, A. et al. Global health perspectives on antibacterial drug discovery and the preclinical pipeline. Nat Rev Microbiol (2025). (https://doi.org/10.1038/s41579-025-01167-w) is an annotated tour by mechanism of current pre-clinical strategies with a focus on projects that have been peer-reviewed by one of the competitive funding processes (e.g., CARB-X). The survey covers both classical direct-acting small molecules as well as the various possible non-traditional strategies (antibodies, phage, etc.).
- Clinical pipeline: Paterson DL: Expert Opin Investig Drugs 2024 (Mar 6:1-17, doi: 10.1080/13543784.2024.2326028) is an excellent survey of the 28 small molecules and 20 non-traditional products currently in Phases 1-3 as candidate therapies for Gram-negative bacteria.
- Clinical pipeline: Butler et al. J Antibiot 2023 (https://doi.org/10.1038/s41429-023-00629-8) is an update through December 2022 of the Butler et al. 2020 J Antibiot paper cited below.
- Clinical pipeline: Neha Prasad, author of the excellent “Leaky Pipeline” paper (AAC 2022, doi:10.1128/aac.00054-22, see also this 14 June 2022 newsletter) has posted on LinkedIn a comprehensive 6-part summary of clinical and corporate antibacterial development news for 2022-2023.
- Clinical pipeline: Theuretzbacher et al. Nat Rev Microbiol 2020 (https://doi.org/10.1038/s41579-020-0340-0) is an extended commentary on the current pipeline by the group who did the WHO 2019 review.
- Clinical pipeline: Butler and Paterson, J Antibiot 2020 https://doi.org/10.1038/s41429-020-0291-8: Not open access, but you can view it online here.
- Commercial view of recently approved drugs: Alan Carr 2020: Newsletter is here. Alan’s regularly updated summary analyzes products approved in the US since 2009
- Non-traditional products: Theuretzbacher and Piddock, Cell Host Microb 2019 (https://doi.org/10.1016/j.chom.2019.06.004) and Rex et al. Nat Commun 2019, https://doi.org/10.1038/s41467-019-11303-9: Focused reviews and discussions of non-traditional products. Go here for a newsletter about these two papers.
- Preclinical pipeline: Theuretzbacher et al. Nat Rev Microbiol 2019 (https://www.nature.com/articles/s41579-019-0288-0) surveys 400+ active preclinical programs.
- Access- and Stewardship-focused reviews
Drug Manufacturing Standards
- On June 14, 2022, the AMR Industry Alliance (AMRAI) published its Antibiotic Manufacturing Standard: Minimizing risk of developing antibiotic resistance and aquatic ecotoxicity in the environment resulting from the manufacturing of human antibiotics. The Standard, facilitated by BSI Standards Limited (BSI, a UK-based standards body), provides clear guidance to manufacturers in the global antibiotic supply chain to ensure that their antibiotics are made responsibly, helping to minimize the risk of AMR in the environment.
- On 6 June 2023, AMRAI and BSI announced the availability of a global Minimized Risk of Antimicrobial Resistance (AMR) certification process that would confirm compliance with the 2022 Standard.
Impact of AMR on cancer therapy
Dear All (unapologetically wonkish … very important material!), Let’s set the scene today by considering two quotes: Prosaic: “The successful treatment of patients with cancer has long depended on the capacity to manage infectious complications.” (Shropshire 2025, cited below) Blunt translation: “Your cancer will be controlled, but then you may die of infection.” (Abdul Ghafur,
Evolving the idea of DTR (Difficult-to-Treat Resistance) to include antibiotic access
Dear All (wonkish alert … lots of details here … take your time and absorb it all!), I’ve written several times about the concept of DTR, or Difficult-to-Treat resistance, that was proposed in 2018 by Kadri et al. Today we will consider an important update from Kadri and colleagues in which the idea of DTR