Dear All,
A key theme of all discussions on Pull incentives is that substantial rewards should go only to molecules with high medical value. We’re still learning to define the idea of high value and today’s newsletter brings together 3 threads that feed into this conversation:
First, we have the idea a priority pathogen list. By my count (amr.solutions Bugs+Drugs webpage), we’ve had six of these so far. The 2017 WHO Bacterial Pathogen Priority List (PPL) is in need of updating and your input is requested! The survey takes ~30 minutes, it autosaves if you pause for coffee, you may get email reminders about finishing the survey if you don’t complete it in one sitting, and your responses will remain anonymous (but there is an option to share your name for acknowledgement of your participation via a delinked form). The survey is open until 20 April 2023 — keeping this list up to date provides vital guidance to policy makers — please make time for it!
Second, ENABLE-2 has launched an open call for molecules with direct-acting systemic antibacterial activity and a novel mode of action targeting the ESKAPE pathogens. For those new to the area, ESKAPE was the original PPL and is still a very useful acronym … to learn more about it, see the links on the amr.solutions PPL summary webpage. At this time, the call is specifically for academics and SMEs in Sweden and publicly funded European universities. The call will remain remain open until 5 May 2023 and you can read more details about how to apply on their website.
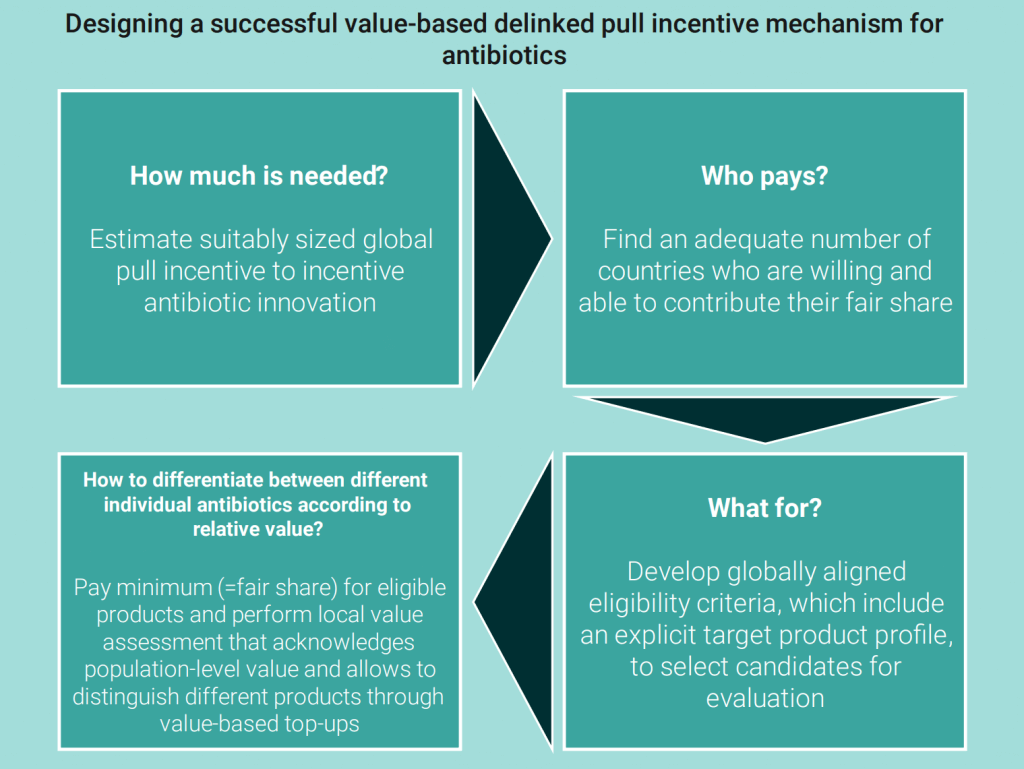
Finally, the UK’s Office for Health Economics recently published the “Incentivising New Antibiotics” Report. This comprehensive report which shares insights from the NICE/NHSE AMR pilot and the considerations behind calculating a country’s fair share of push/pull mechanisms. The UK Pilot is / has been a ground-breaking effort to articulate measures of antibiotic value and is discussed in many newsletters. In particular, I would recommend the discussions of the pilot’s pilot point-scoring scheme in “UK Antibiotic Subscription Pilot Implies Pull Incentive Of Up To $4b Across The G20” and the data behind the value of two initially selected antibiotics in “UK Pull (Netflix) Pilot: STEDI-Based QALY Value Of New Antibiotics Implies Societal Value > 10m GBP/Year!” You should also consider the related discussions of economics in “What Does An Antibiotic Cost To Develop? What Is It Worth? How To Afford It?” and antibiotic value in “Impact Of PASTEUR: 9.9m Lives Saved, ROI Of 125:1”.
The new OHE report is visually stunning and the information well presented. The figure below from page iv is great summary of the key steps that have occupied our thinking for the past several years:
To quote the report, the UK’s “pilot showed an example of what is possible, but in taking a theoretical model and seeking to make it real, it has highlighted many implementation challenges and also conflicting views about the precise function and requirements for pull incentives to combat AMR.”
As always, I highly recommend you read it yourself and pass it along to anyone you think might find it interesting. This report is so elegantly presented that it strikes me as an easy read even for those outside our AMR community.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here)
- NIAID BAA with a biodefense focus: Vaccines, Therapeutics, and Diagnostics are all in scope. See this newsletter for details; various due dates through 11 April 2023.
- [UPDATED – New application round] CARB-X again has an open-round for funding applications with a deadline of 1 May 2023. Applications are sought for any of 3 themes (oral products, vaccines for neonatal sepsis, gonorrhea products) as described in this newsletter!
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 14 Apr 2023 (Copenhagen, Denmark; 3-6.30p CEST): ECCMID and the Global Leaders Group on AMR will jointly sponsor a symposium entitled “Forging partnerships between science and policy in Antimicrobial Resistance (AMR).” Go here to register.
- 15-18 Apr 2023 (Copenhagen, Denmark): 33rd ECCMID. Go here for details and to register.
- 26 Apr 2023 (virtual, noon-1.30p CET): Webinar entitled “WHO Human health AMR research agenda” from WHO’s series entitled “WHO Global Webinar Series to Support Implementation of National Action Plans on Antimicrobial Resistance (AMR).” Go here to register.
- 8-12 May 2023 (Lisbon, Portugal): 41st Annual Meeting of the European Society for Paediatric Infectious Diseases. Go here for details.
- 3-5 Jul 2023 (Tours, France): 9th Symposium on Antimicrobial Resistance in Animals and the Environment (ARAE). Sponsored by INRAE (French National Research Institute for Agriculture, Food, and Environment, itself a merger of merger of INRA, the French National Institute for Agricultural Research, and IRSTEA, the French National Research Institute of Science and Technology for the Environment and Agriculture), this conference has been running since 2005. Go here for details.
- [NOW OPEN FOR APPLICATIONS!] 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.