Dear All:
The recent publication of an exceptionally good plain-language summary of the AMR problem in Rolling Stone (yes, you read that correctly!) prompts today’s 3-part journey into the way(s) that war contributes to the threat of resistant superbugs. We’ve summarized the story in outline form — please explore the references for further details. And (wonkish alert!), the story goes from relatively non-technical language to technical language. With that, we’re off to the tour!
- “Could a Conflict-Borne Superbug Bring on Our Next Pandemic?”
- Author and source: Eli Cahan, 21 July 2024, Rolling Stone
- (behind a paywall, apologies): https://www.rollingstone.com/politics/politics-features/war-zone-conflict-bacteria-pandemic-1235064261/
- (28Oct2024 addendum and not behind a paywall!): Podcast discussion with Dr. Cahan about AMR, this article, and his summary of AMR as “the climate change of medicine.” It’s podcast #7 in the the “Infectious Conversations” series from Partnership to Fight Infectious Disease (PFID).
- (25 Nov 2024 addendum): And going more broadly than conflict-related AMR, Carine Naim of Médecins Sans Frontières (MSF) shared the excellent MSF report entitled The Broken Lens: Antimicrobial Resistance in Humanitarian Settings (Nov 2024) that discusses the way humanitarian crises (conflict, climate change, displacement, underfunded health systems) drive AMR. On this theme, you might also be interested in her paper entitled The role of humanitarian actors in global governance for AMR (Lancet Global Health 12:e1752 – e1753, 2024).
- (26 Nov 2024 addendum): The pace of stories about conflict-related AMR is astonishing! See now Modern Warfare Is Breeding Deadly Superbugs. Why? from the New York Times (26 Nov 2024). It’s a superb long read.
- Summary: A vivid telling of the stories of 3 soldiers (two Ukrainian, one American) who struggle with infections due to highly resistant Acinetobacter spp. and Klebsiella pneumoniae. Two survive, one dies.
- Key messages:
- The detritus of war (bomb fragments, city wreckage) creates heavy-metal-laden cesspools that encourage development of resistance.
- Traumatic injuries provide an instant introduction of environmental bacteria into wounds and (no surprise) comprehensive treatment is usually not immediately available.
- Further, shrapnel in injured soldiers can carry these bacteria and (key!) often cannot be fully removed, thus creating reservoirs from which superbugs are carried into hospitals and the general population.
- The article reminds us of the term “Iraqibacter” (see below) and calls for action on the PASTEUR Act.
- “Six Extensively Drug-Resistant Bacteria in an Injured Soldier, Ukraine”
- Authors and source: McGann PT et al., August 2023, Emerging Infectious Disease
- https://wwwnc.cdc.gov/eid/article/29/8/23-0567_article
- Summary: After being badly burned, a soldier in the Ukraine is found to be simultaneously carrying six different highly resistant bacteria!
- Key messages:
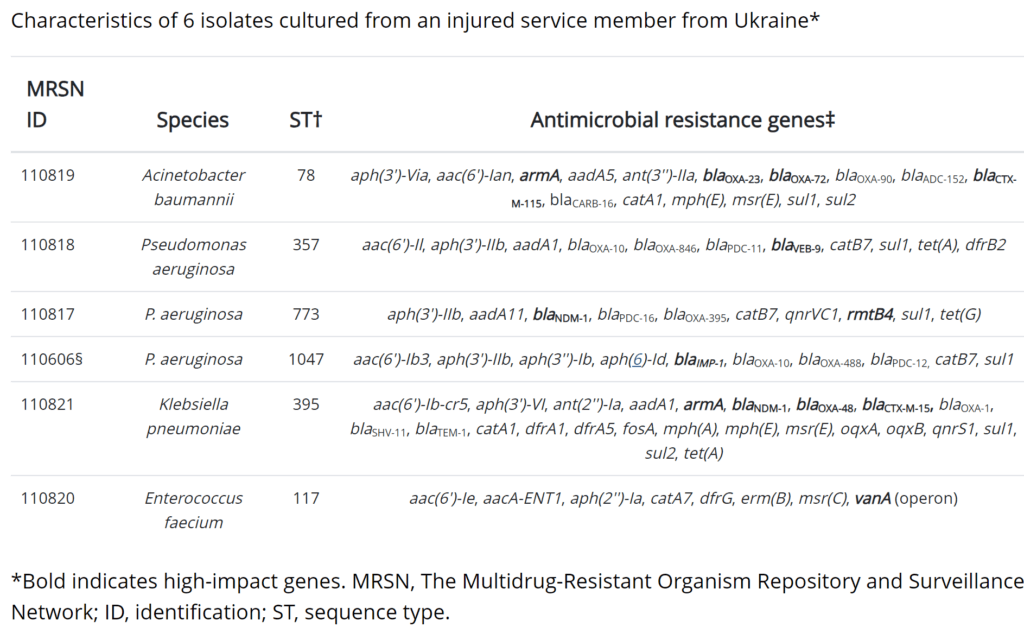
- Upon being moved to a US military hospital in Germany, the injured soldier is found to be simultaneously infected with six (6!) XDR pathogens: Acinetobacter baumannii, P. aeruginosa (3 different strains), Klebsiella pneumoniae, and Enterococcus faecium.
- These organisms are together carrying 79 different resistance genes (mean 13/organism, range 8-24, see the figure below our signatures).
- Each organism carries at least one high-impact resistance gene (e.g., genes for carbapenem resistance, vancomycin resistance, or similar). The idea of “high-impact” is further explored just below.
- Related non-technical papers
- An excellent 2 Oct 2023 plain language discussion of the McGann EID 2023 paper by Andrew Jack in the Financial Times. You might also enjoy reprising his excellent 5-minute video explainer of the AMR problem!
- An 11 May 2008 brief NPR report about a soldier with a blast-related injury that was complicated by highly resistant Acinetobacter, dubbed Iraqibacter in the article, is just one anecdote out of many regarding such incidents among soldiers returning from Iraq and Afghanistan.
- Moussally et al. provide a similar perspective in their recent paper “Antimicrobial resistance in the ongoing Gaza war: a silent threat”, Lancet 2023.
- And let’s not forget that resistance is also driven by other factors: US CDC recently updated their estimates of the US burden of resistant pathogens and found that a group of six bacterial antimicrobial-resistant hospital-onset infections (two graded as Urgent threats, four graded as Serious, see the 2019 threat report for the scoring scheme) increased by a combined 20% during the COVID-19 pandemic and have remained above pre-pandemic levels in 2022. Relatedly, the number of cases of infection deu to C. auris (a resistant fungus) has now risen 5-fold since 2019.
- 29 July 2024 Addendum: The 22-23 May 2024 meeting of PACCARB devoted its 23 May morning session to the topic of combat-related drug resistance. See page 3 of the agenda and then follow along in the video on YouTube.
- “Assessment of global health risk of antibiotic resistance genes”
- Authors and source: Zhang Z et al., 2022, Nature Communications
- https://www.nature.com/articles/s41467-022-29283-8
- Summary: Metagenomic sequencing of 4,572 samples from around the world found 2,561 distinct antibiotic resistance genes (ARGs). Of these ARGs, ~25% (~600) were judged to pose a human health risk.
- Key messages:
- Zhang et al. did metagenomic sequencing on 4,572 samples obtained globally by sampling six habitats: air, aquatic, terrestrial, engineered, humans, and other hosts. Metagenomic sequencing approaches take an entire sample and sequence everything all at once without trying to identify individual organisms.
- Zhang et al. then searched the resulting sequencing database for antibiotic resistance genes (ARGs).
- Knowing that all resistance genes are not created equal, Zhang et al. ranked the ARGs based on human accessibility (was the sample in/near a human habitat), mobility, human pathogenicity, and clinical relevance. Based on the location of the sample, socioeconomic factors such as access to adequate sewage treatment and clean water were also assessed.
- Their scoring system suggested that ~25% of the ARGs were high risk.
- Comments and related papers
- There is no perfect way to score the risk of ARGs, but it is interesting to see this approach to handling metagenomic data.
- McGann EID 2023 does not state criteria for “high impact” but their choices (the bold-faced gene in the table below) suggest that they are the genes most likely to be responsible for resistance to the most obvious therapeutic choices.
- A prior paper by Martinez, Coque, and Baquero (“What is a resistance gene? Ranking risk in resistomes”, Nat Rev Microbiol 2015, suggested a 7-level scoring risk scheme for metagenomic data that involves the source and mobility associated with ARGs..
- It only applies at the organism level, but another good example of this is found in prior newsletters about MDR/XDR vs. DTR (Difficult-to-Treat Resistance) — start with this 13 Jan 2019 newsletter if this is new to you.
Wow! The idea that the metallic debris of war could itself drive resistance is both horrific and fascinating. As Patty taught me while we were writing this newsletter, “Genes for resistance to heavy metals were found in the very earliest studies of plasmid-borne resistance. An early example would be the 1977 AAC papers by Nakahara et al. entitled ‘Mercury Resistance and R Plasmids in Escherichia coli Isolated from Clinical Lesions in Japan’ and ‘Linkage of Mercury, Cadmium, and Arsenate and Drug Resistance in Clinical Isolates of Pseudomonas aeruginosa’. The titles are pretty self-explanatory and it’s fair to say that heavy metal resistance appears to be an ancient mechanism.” Yow! For a recent review, see “Heavy metal-induced selection and proliferation of antibiotic resistance: A review”, Vats P et al, J Appl Microbiol 2022.
And the idea that retained shrapnel carrying bacteria would be long-term nidus for spread is obvious once you realize the difficulty with removing shrapnel.
Summing up, this is all the more reason to ring the global alarm for the need for new drugs! They are really hard to find (9 Apr 2024 newsletter, “48,015 → 0: Antibacterial Discovery Is Hard. Really, Really Hard”), the current pipeline remains thin (15 Jun 2024 newsletter: “WHO Antibacterial Pipeline Review: Update Thru 31 Dec 2023”), and the perverse economics of antibiotics lead to market failure once approved (19 Mar 2024 newsletter: “Tragedy Of The (Antibiotic) Commons: A True Market Failure”)!
Our best approach to fixing the pipeline in a durable way remains adequate Pull incentives! The UK model has shown us that it can be done … and now we need the PASTEUR Act in the US, the proposed TEV-based Pull from the European Commission as well as the ideas on Pull from Switzerland, Canada, and Japan.
Onward!
With all best wishes, John & Patricia
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Patricia Bradford, PhD | Antimicrobial Development Specialists, LLC| pbradford@antimicrobialdev.com. All opinions are my own.
——
From McGann EID 2023: Table describing the 6 XDR pathogens in the injured soldier:
- Vivli has announced the 2024 Vivli AMR Surveillance Data Challenge. This particular challenge is funded by GARDP, Paratek, Pfizer and Vivli and aims to encourage and support the innovative re-use of surveillance data shared by GSK, Johnson & Johnson, Pfizer, Shionogi, Paratek and Venatorx that are now available in the Vivli AMR Register. This is 2nd AMR data challenge from Vivli — see the 20 Oct 2023 newsletter for a discussion of the outcome of this first challenge. This 2nd challenge offers monetary prizes, including travel funding to attend ESCMID Global or ASM Microbe in 2025 (if an abstract is accepted), and a new AMR Student Innovation Award. The deadline for expressions of interest is 28 July 2024. For more details, go here. NEW: There is a 23 Jul 2024 (virtual, 11a-12.30p EDT, 5p-630p CEST) GARDP REVIVE webinar that will discuss the challenge.
- Environmental AMR issues, anyone? ICARS has call open through 1 Aug 2024 for “projects in the public health sphere that aim to mitigate the evolution and transmission of resistance in the natural or built environment.” Grants are available of up to $800k for up to 4 years. Go here for details; for questions and submissions, write to RFP_EDAR@icars-global.org. Applicants should also refer to “Mitigating antimicrobial resistance (AMR) using implementation research: a development funder’s approach” from JAC 2023 (https://doi.org/10.1093/jacamr/dlad031).
- The AMR Industry Alliance have announced the 2024 edition of their ongoing annual series of stewardship prizes. Applications for innovative approaches to AMR stewardship are sought from public, private or not-for-profit health care organization or institution operating in an LMIC. This year’s deadline is 1 Sep 2024. Go here for details.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X has open calls at intervals that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to register!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
Upcoming meetings of interest to the AMR community:
- 22 Aug 2024 (virtual, 11a-12.30p EDT, 5p-630p CEST): GARDP REVIVE Webinar entitled “Exploring non-traditional antimicrobials: Insights from three cases.” Go here for details and to register. If non-traditional approaches interest you, please do be sure to review the challenges that are raised in the papers discussed in the 6 Aug 2019 newsletter entitled “Non-Traditional Antibiotics: A Pipeline Review And An Analysis Of Key Development Challenges.” Developing non-traditional products is MUCH harder than you might expect … it is important to know the issues!
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.