Dear All (wonkish alert … lots of details here … take your time and absorb it all!),
I’ve written several times about the concept of DTR, or Difficult-to-Treat resistance, that was proposed in 2018 by Kadri et al. Today we will consider an important update from Kadri and colleagues in which the idea of DTR is adjusted to consider the implications of limited antibiotic access.
We will take the conversation in 3 parts: (1) What is DTR?, (2) What adjustments to the definition of DTR were found to be needed?, and (3) What does the new idea of DTR Index teach us? Off we go!
First stop: What is DTR?
- The original idea of DTR was developed for Gram-negative bacteria and was built on ideas that are the basis for the definitions of MDR, XDR, and PDR (Multi-drug resistance, eXtensive-Drug Resistance, Pan-Drug Resistance):
- Carbapenem resistance (CR): Resistance to any one of imipenem, meropenem doripenem, or ertapenem
- Extended-spectrum cephalosporin resistance (ECR): Resistance to any one of ceftazidime, cefepime, ceftriaxone, or cefotaxime
- Fluoroquinolone resistance (FR): Resistance to any one of ciprofloxacin, levofloxacin, or moxifloxacin
- Reference: Magiorakos 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. CMI 2012;18:268-81.
- With these ideas in your head, DTR for Gram-negatives is CR plus ECR plus FR
- Note carefully: The definition does NOT include any of the BL-BLI (beta-lactam – beta-lactamase inhibitor combinations)
- A good place to start if these definitions and ideas are new to you:
- 5-minute YouTube explainer about the ideas of UDR-MDR-XDR and DTR
- Newsletters about the ideas of DTR:
- References:
- Kadri et al. 2018: Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clinical Infectious Diseases. 2018;67(12):1803-14 (https://doi.org/10.1093/cid/ciy378).
- Kadri et al. 2019: External Validation of Difficult-to-Treat Resistance Prevalence and Mortality Risk in Gram-Negative Bloodstream Infection Using Electronic Health Record Data From 140 US Hospitals. Open Forum Infect Dis. 2019;6(4):ofz110 (https://doi.org/10.1093/ofid/ofz110).
So, why does DTR matter? Well, at the time of the creation of the definition of DTR, there were very few good antibiotic choices for a DTR pathogen. The choices that did exist were generally more toxic and otherwise harder to use (e.g., an aminoglycoside, a class with well-known risk of kidney injury). Significantly, the need to select an (older) antibiotic that covered a DTR pathogen was shown to be associated with increased mortality. Here’s a good example of this from Kadri 2019:
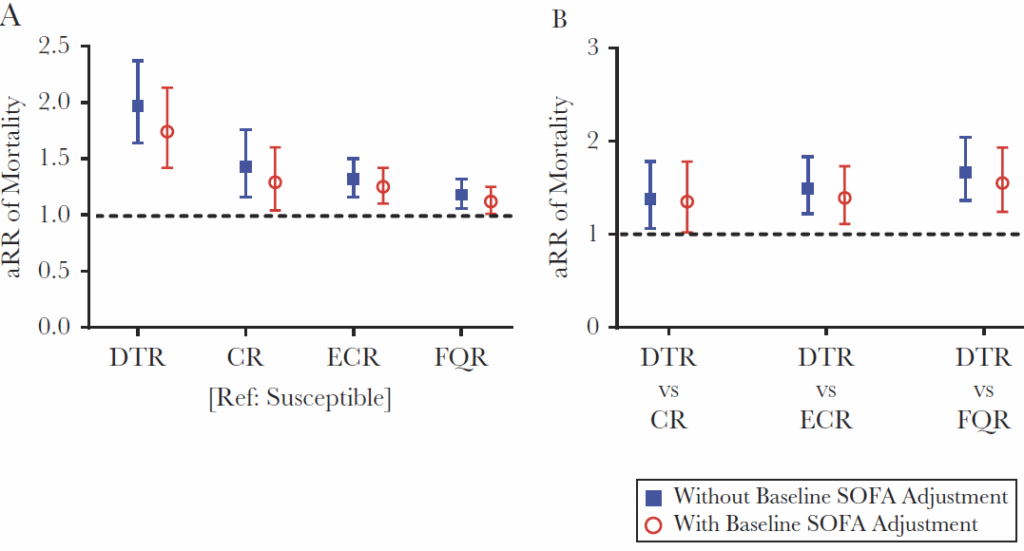
In each figure, the y-axis is RR (Relative Risk) of mortality. In the left-hand panel, all RR values use a non-DTR antibiotic as the reference category (the denominator in the ratio) and thus any value > 1.0 means that mortality is worse. As you can see, the RR is highest for DTR vs. non-DTR but there is also an increment for being just CR, ECR, or FR. In the right-hand panel, DTR is compared with each of its components. In both panels, the data are shown with and without and adjustment for severity of illness (SOFA, sequential organ failure assessment). The increased mortality of DTR is readily apparent in all comparisons.
—
Second stop: What adjustments to the definition of DTR were found to be needed?
As reviewed in detail in Walker 2025 (Using a difficult-to-treat resistance index to gauge imbalance between countries’ antibiotic resistance prevalence and access to antibiotics: a scoping review and concept proposal. Clin Micro Infect https://doi.org/10.1016/j.cmi.2025.02.029), several things became apparent as the idea of DTR was used (all quotes are from Walker 2025):
- First, DTR “helped change the paradigm from
- ‘How many antibiotics or antibiotic categories are inactive against a pathogen?’ to
- ‘Is there even one safe and effective first-line agent active in vitro to treat the patient?’”
- Second, DTR was repeatedly confirmed as a predictor of mortality.
- Third, it was realized that the DTR definition “does not consider the recent introduction of newer antibiotics (e.g. ceftazidime/avibactam, ceftolozane/tazobactam) with a broad antimicrobial spectrum of activity and improved side effect profiles vs. older reserve agents against DTR pathogens (e.g. polymyxins).”
- Thus, and if available, these newer antibiotics do not meet the spirt of the idea that DTR is a requirement to use an older and more toxic antibiotic.
- Finally, that phrase “if available” emerged as a very large factor as the definition “assumes access to all beta-lactam and fluoroquinolone antibiotics.”
It’s that bit about availability that really creates an issue … and with that, the penny drops! As we have discussed in several recent newsletters most countries only get access to newer antibiotics late, if at all. If this is new to you, here are some good newsletters to review on this topic:
- 19 Aug 2021: “Ex-US new antibiotic access: Multi-year delays, even in Europe!”
- 25 Sep 2023: “Manufacturing underpins both access and stewardship: Cefiderocol as a case study”
- 1 Dec 2024: “The 6 meanings of ‘Lack of Access’ (UNSLAP)”
- 23 May 2024: “Lancet: 10-20-30 targets to address AMR by 2030”
- 7 Apr 2025: “UNSLAP: You reach for the antibiotic … and it’s not there!”
- 14 Apr 2025: “Manufacturing underpins access in LMICs: An update on cefiderocol”
Stated differently, the existence of an approved antibiotic doesn’t mean it is approved and available to you. Indeed, you (personally) might face a situation wherein the pathogen causing your infection is effectively resistant to everything available to you.
With these ideas in mind, Kadri and colleagues propose the idea of a DTR index that intersects DTR with availability by changing the definition of DTR from
- ‘resistant to all first line, highly safe and effective antibiotics’ to
- ‘resistant to all first line, highly safe and effective antibiotics available in that country at that time.’
A visual way to appreciate the way that DTR (if everything is available) and DTR index (the reality in which DTR expands or shrinks based on local access) is provided by Walker 2025:
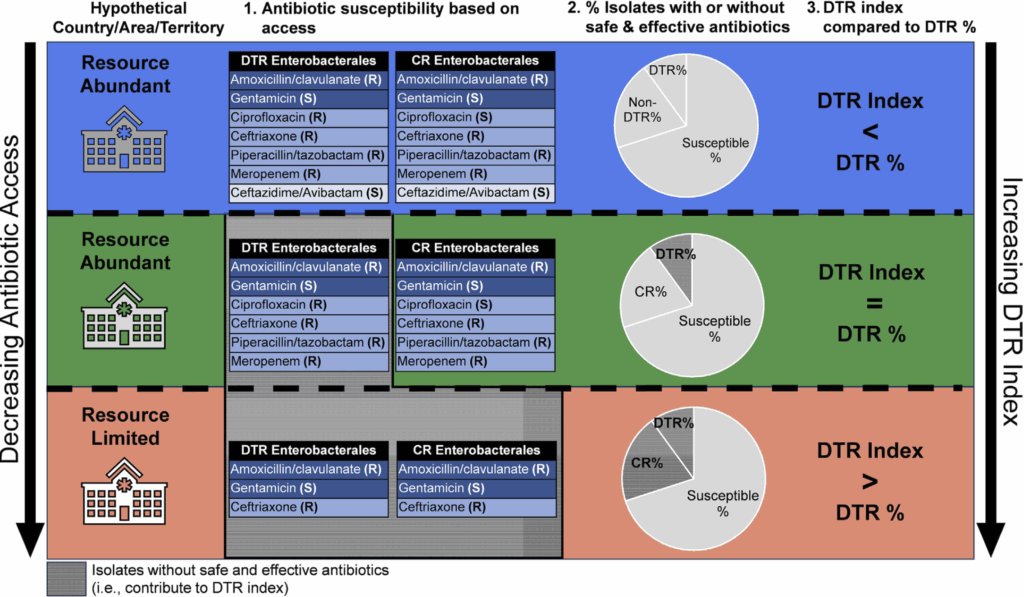
Look closely at the 3 rows.
- In the uppermost row, all antibiotics, including a newer BL-BLI (ceftazidime-avibactam) are available — and the DTR index is smaller (zero) relative to the standard DTR %.
- In the middle, all the DTR-defining antibiotics are available, but not the BL-BLI … and the DTR index is identical to the DTR%.
- Finally, the bottom row is situation where only a few antibiotics are available … and hence the DTR index is more than the DTR %.
Third stop: What does the new idea of DTR Index teach us? The DTR Index is an interesting and valuable build, but issues remain. First, and like DTR itself, it requires a lot of data on local susceptibility patterns. Second, it requires good knowledge of what is available in a given location. Third, I find the nomenclature a bit opaque … I wondered if “theoretical DTR” and “locally adjusted DTR” might be easier to quickly communicate.
But, those are minor quibbles relative to the power of the ideas of DTR and DTR Index. If the needed drug is not available to you, it may as well not exist. Thus, calls for access (e.g., the superb papers by Laxminarayan and colleagues summarized in the 23 May 2024 newsletter entitled “Lancet: 10-20-30 targets to address AMR by 2030”) need to be translated into action. But, it will be slow going as shown by the substantial effort required just to enable access in LMIC countries to a single new antibiotic (14 Apr 2025: “Manufacturing underpins access in LMICs: An update on cefiderocol”).
And one other idea to note in passing (a newsletter for another day, I think) Kadri & colleagues have also shown that newer drugs are not always used, even when available (see Strich 2024: Assessing Clinician Utilization of Next-Generation Antibiotics Against Resistant Gram-Negative Infections in U.S. Hospitals: A Retrospective Cohort Study. Ann Intern Med. 2024;177:559-72, https://doi.org/10.7326/M23-23 and the excellent associated commentary by Howard-Anderson and Boucher: New Antibiotics for Resistant Infections: What Happens After Approval? Annals of Internal Medicine. 2024:177:674-5, https://doi.org/10.7326/M24-0192). You can probably guess the answer to the question posed by the title of the commentary!
—
Whew! It may sound like it is impossible to address the issues highlighted by the ideas of DTR and DTR Index. But, I think I’d say instead that “well begun is half done.” Yes, we definitely need new drugs for bad bugs … and then we need to make them available … and then we need to use them when needed … and at least now we are talking about it!
Many thanks to Kadri & colleagues for the ideas coming out of their ongoing research! All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- The Trinity Challenge on Community Access to Effective Antibiotics is a £1 million innovation competition that seeks applications to answer the question: “How can data and technology improve stock control and/or reduce the use of substandard and falsified oral antibiotics for community use in low- and middle-income countries?” Applications are open at this website until 24 April 2025.
- HERA posted a call on 24 Mar 2025 entitled “Development of a Rapid Point-of-care Antimicrobial Susceptibility Testing Diagnostic Medical Device (HADEA/2025/CPN/0006).” In it, they call for tenders under which they will provide up to 13m EUR for development of a point-of-care diagnostic medical device that can provide antimicrobial susceptibility results on the bacteria or fungi causing an infection in humans, within one hour or less from subject sample collection, and ideally, to also allow for pathogen identification. To apply, you must submit a request to participate by 12 May 2025; selected candidates who met the eligibility criteria will be able to submit a full technical tender. Go to the EU Funding and Tenders Portal to apply; see also the 19 Feb 2025 newsletter for details.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X will have two calls during 2025 that span two areas: (i) Small molecules for Gram-negatives (the focus is on Pseudomonas aeruginosa) and (ii) Diagnostics for typhoid (the focus is diagnosis of acute infections in 60 minutes or less). See this 26 Feb 2025 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. The first cycle will accept expressions of interest during the window 16-30 April 2025; the 2nd round will be open 1-12 Dec 2025.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are BEAM’s AMR Conference and GAMRIC (formerly, the ESCMID-ASM conference series). Hope to see you there!
- [UPDATED LOCATION – To maximize options for global attendees, the conference location is now central London, UK] 1-3 Oct 2025 GAMRIC, the Global AMR Innovators Conference (London, UK). Formerly the ESCMID-ASM Joint Conference on Drug Development for AMR, this meeting series is being continued under the joint sponsorship of CARB-X, ESCMID, BEAM Alliance, GARDP, LifeArc, Boston University, and AMR.Solutions. The ongoing series will continue the successful format of prior meetings with a single-track meeting and substantial networking time (go here to see details of the outstanding 2024 meeting). Registration will open on 5 May 2025; in the interim, the preliminary agenda can be found at that same link (https://www.gamric.org/). The meeting will be limited to approximately 300 attendees, so please be sure to register promptly to avoid disappointment! The abstract submission window will run 5 May to 13 June and an application round for travel grants is expected to run in a similar time frame.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society of America. Details pending; go here for the general meeting website.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference. Sponsored by the BEAM Alliance, the 9th AMR Conference has just concluded and it’s again been an excellent meeting! Please mark your calendar for next year. You can’t register yet, but details will appear here!
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. You can’t register yet, but you can go here for details on the outstanding 2025 meeting.
Upcoming meetings of interest to the AMR community:
- 30 Apr 2025 (virtual, 10-5.15p ET): Presented by Sepsis Alliance, the Sepsis Alliance AMR Conference is 1-day virtual discussion of AMR- and sepsis-focused diagnostics, therapeutics, and advocacy. Go here for details and to register.
- 19-23 June 2025 (Los Angeles): ASM Microbe, the annual meeting of the American Society for Microbiology. Go here for details.
- 10-13 Sep 2026 (Lisbon, Portugal): 6th ESCMID Conference on Vaccines. Go here for details.
- [UPDATED LOCATION] 1-3 Oct 2025 GAMRIC, the Global AMR Innovators Conference (London, UK; formerly the ESCMID-ASM Joint Conference on Drug Development for AMR). See list of Top Recurring meetings, above..
- 11-19 Oct 2025 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2025 will be the 9th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications should open ~March 2025 — go here for more details.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025. See list of Top Recurring meetings, above.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference sponsored by the BEAM Alliance. See list of Top Recurring meetings, above.
- 8-13 Mar 2026 (Renaissance Tuscany Il Ciocco, Italy): 2026 Gordon Research Conference (GRC) entitled “Antibacterials of Tomorrow to Combat the Global Threat of Antimicrobial Resistance.” A Gordon Research Seminar (GRS) will be held the weekend before (7-8 Mar) for young doctoral and post-doctoral researchers. Space for the GRS and the GRC is limited; for details and to apply, go here for the GRC and here for the GRS.
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
Self-paced courses, online training materials, and other reference materials:
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.
- GARDP’s REVIVE website provides an encyclopedia covering a range of R&D terms, recordings of prior GARDP webinars, a variety of viewpoint articles, and more! Check it out!
- GARDP’s https://antibioticdb.com/ is an open-access database of antibacterial agents.
- The CARB-X website provides a range of recordings from its webinars, bootcamps, and more. A bit of browsing would be time well spent!
- British Society for Antimicrobial Chemotherapy offers an eLearning section: Education – The British Society for Antimicrobial Chemotherapy.