Dear All (and with a wonkish alert!),
The idea of more (and better) vaccines to prevent (or even be part of treating) bacterial infections has long intrigued. We certainly do have several good bacterial vaccines, but why not more?
As part of this ongoing discussion, I really enjoyed a paper that came out back in December ’24 about the impact of the vaccine modality itself. Here are the links you need:
- The paper that triggered this newsletter: Plotkin SA, Robinson JM, Fitchett JRA, Gershburg E, Vaccine Development Should Be Polytheistic, Not Monotheistic, Clinical Infectious Diseases, 79(6):1518–1520, https://doi.org/10.1093/cid/ciae460
- An excellent brief discussion of mRNA vaccines for bacterial infections: Bergstrom C, Fischer NO, Kubicek-Sutherland JZ, Stromberg ZR. mRNA vaccine platforms to prevent bacterial infections. Trends Mol Med. 2024;30(6):524–6 (doi.org/10.1016/j.molmed.2024.02.013).
- Prior newsletters about antibacterial vaccines
- 9 Oct 2018 newsletter: “Vaccines for AMR / A major review of R&D opportunities is a discussion of a Wellcome-funded report entitled “Vaccines to tackle drug-resistant infections: An evaluation of R&D opportunities.”
- 29 Mar 2021 newsletter: “Vaccines to turn back the tide of antimicrobial resistance is a discussion of WHO’s Immunization Agenda 2030: A Global Strategy to Leave No One Behind,
- 26 Nov 2024 newsletter: “NIAID/DMID call for antibacterials, antifungals, vaccines, & diagnostics” is exactly what it sounds like
- 12 Dec 2024 newsletter: “Mirror Bacteria: An AMR threat of unprecedented magnitude” covers (briefly) the possible role of new vaccines in combatting mirror bacteria.
So, what’s the idea behind polytheism vs. monotheism? Well, the paper by Plotkin et al. is a brief (3-page) high-level summary of the strengths and weaknesses of various vaccine technologies. A simple summary might be
- mRNA vaccines are very flexible but expensive and do not (so far) give consistently durable immunity.
- Other modalities may be less flexible but are often more durable and less costly.
The more detailed summary is in their Figure 1:
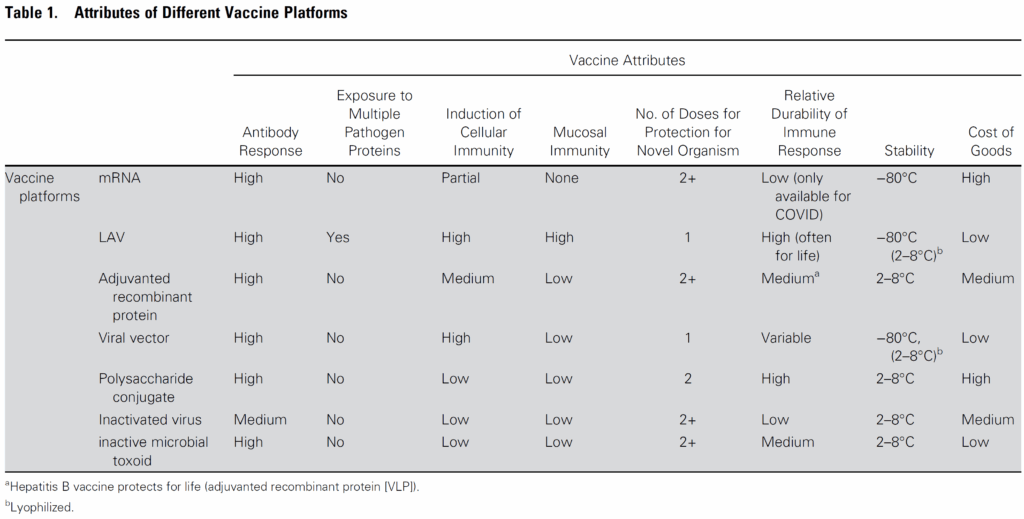
Walking through the table,
- The mRNA vaccines have outstanding flexibility but it’s now clear that they don’t produce long-term immunity (even with multiple doses) and they also don’t induce immunity at mucosal surfaces. In addition, they are very expensive to produce and require an ultra-low temperature cold chain to deliver and use.
- LAV (live-attenuated vaccines) are inexpensive and produce durable immunity (including at mucosal surfaces) with a single dose. LAV are also notable for producing effects against variations of the pathogen and hence are protective even when mutations occur.
- Adjuvanted recombinant proteins, viral vectors, and inactivated viruses produce durable immunity at a low cost but may require more than one dose
So, the authors of this (mostly virus-focused) paper argue that “although the rapid success of mRNA vaccines against COVID-19 is a remarkable achievement, long-term control of COVID and other infections may depend on older technologies.”.
Stated differently, diversity and complementary approaches (polytheism rather than monotheism) are important.
And what is the implication for antibacterial vaccines? Well, here we turn to the related paper by Bergstrom et al. in which we have a good discussion of the strengths and weaknesses of mRNA as a platform for bacterial vaccines:
- The discussion necessarily begins by reviewing the fact that mRNA vaccines work by providing the instructions (mRNA) for your body to synthesize a protein that is then treated by your immune system as an antigen … and thus an antibody response is induced.
- So, one obvious limitation is that this approach will not work if protective antibodies are best induced by exposure to non-protein antigens (e.g., polysaccharides). Some very important vaccines (e.g., those for pneumococcal pneumonia and meningococcal meningitis) are polysaccharide-based and hence will never be amendable to conversion to mRNA technology.
- At a deeper level, the success with mRNA-based vaccines viruses relies on the fact that an antibody response to a single protein antigen can often effectively neutralize the virus. And the neutralizing antibody titer alone is a good correlate of protection — the higher the level, the greater the protection
- On the other hand, bacteria are more complex and often use multiple mechanisms to evade the host immune response — thus an antibody to just one antigen may be insufficient to stop (or even slow down) the invading bacteria. And from the developer’s standpoint, picking out a best (or even just plausible) antigen is difficult given the many antigens displayed by bacteria.
- Some of these issues might be overcome with multivalent mRNA vaccines (i.e., provide the coding for more than one antigen) or combination approaches (mRNA with something more conventional), these approaches have limitations, especially for complex bacteria for which mechanism of protection are not fully understood.
Efforts are being made to overcome these issues and Bergstrom et al. provide this excellent figure summarizing current efforts to apply mRNA to bacterial vaccines:
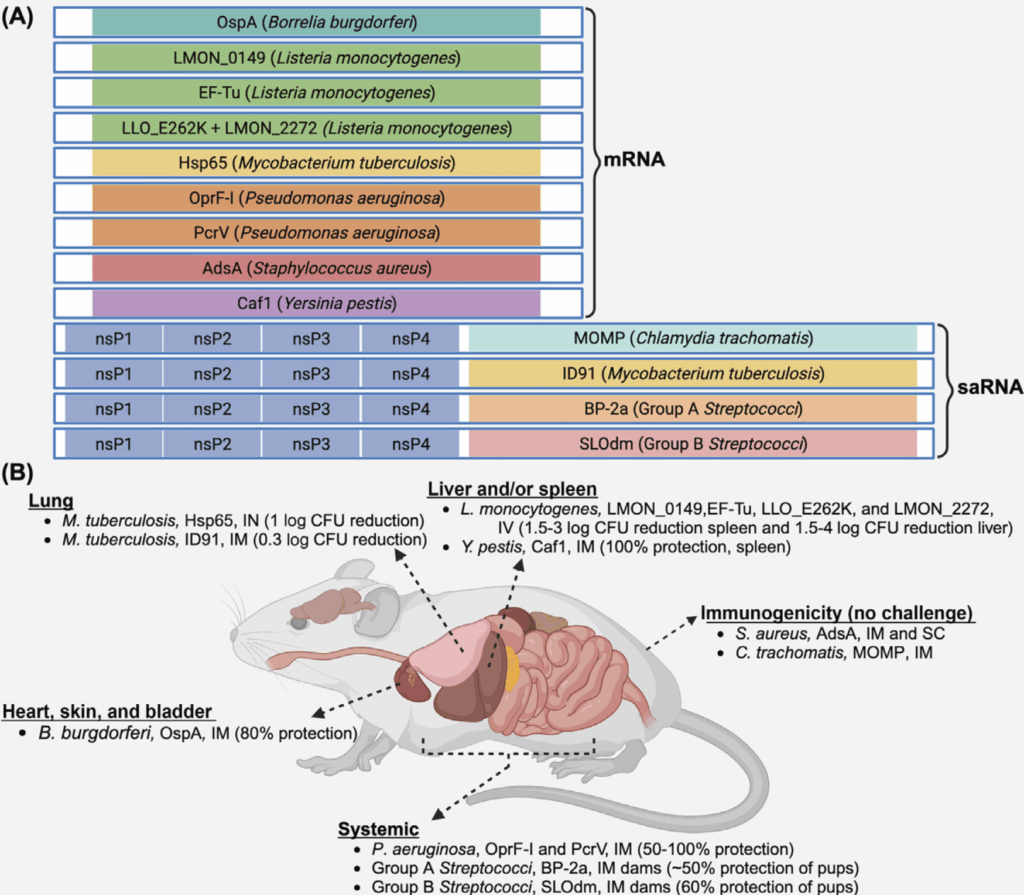
This figure is worth a bit of study as it also introduces the idea of saRNA (self amplifying RNA) vaccines that are composed of the mRNA encoding the antigen plus another mRNA encoding a replicase enzyme that will enable the RNA to replicate within the cell. The advantage of this approach is that you need a lower amount of initial RNA, as this will be replicated after immunization.
Impressive! Reading these papers also made me re-review the excellent figure from that Wellcome report showing need (y-axis) vs. probability of success (x-axis) — some targets are just very, very tough:
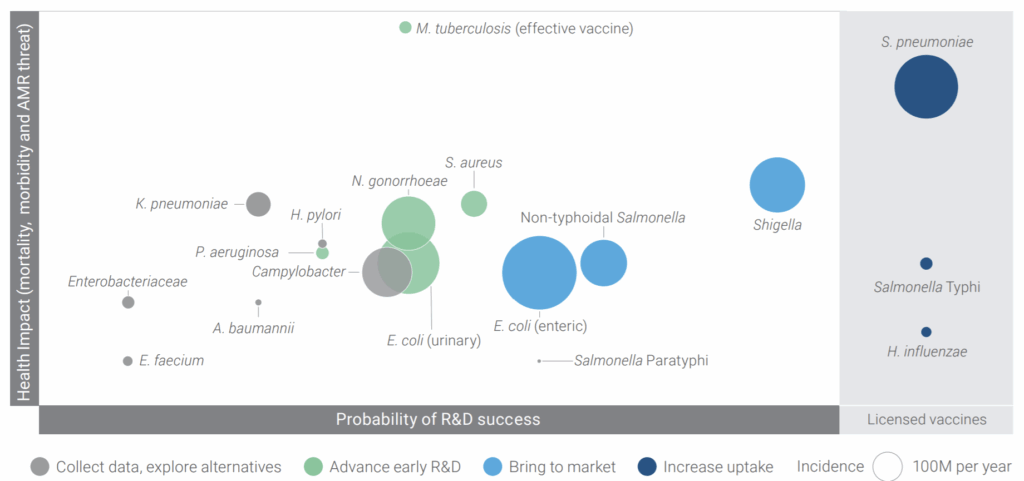
And so, there you have it. Polytheism vs. monotheism and the challenge of vaccines. And make no mistake, vaccines are high risk: As an example, the 13 Feb 2025 announcement by Sanofi of a failed Phase 3 trial of a vaccine for extraintestinal pathogenic E. coli (EPEC). And then, of course, there are the many failed efforts to develop a vaccine for infections due to S. aureus (20 Dec 2018 newsletter; “Ouch! Pfizer terminates S. aureus vaccine trial due futility”) and the 3Q2024 termination by GSK of its vaccine for gonorrhea (GSK4348413), just to name two more bacterial species for which a vaccine would be a wonderful thing!
And thinking more widely, this is also reminiscent of challenge of antibacterial R&D in general … my experience has been that locking in on a single pathway is a risky and that diversity is your friend. CARB-X has been doing a great job of creating a portfolio with a very wide spread of risk in the projects and I want to see this continue!
Meditatively yours (and with thanks to CARB-X’s Vega Masignani for helpful critiques of this newsletter),
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- [NEW] NNF (Novo Nordisk Foundation) have announced their “Challenge Programme 2026 – Unravelling the Pathways of Human Invasive Fungal Diseases. The call seeks applications from EU-centered consortia (global partners are possible) for research in 4 areas: (i) fungal virulence factors, (ii) host-pathogen interactions, (iii) mechanisms of anti-fungal resistance, and (iv) fungal disease markers. Applications are due by 8 Oct 2025. Go here for details.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X will have two calls during 2025 that span two areas: (i) Small molecules for Gram-negatives (the focus is on Pseudomonas aeruginosa) and (ii) Diagnostics for typhoid (the focus is diagnosis of acute infections in 60 minutes or less). See this 26 Feb 2025 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. The first cycle is now closed (it ran16-30 April 2025); the 2nd round will be open 1-12 Dec 2025.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are BEAM’s AMR Conference and GAMRIC (formerly, the ESCMID-ASM conference series). Hope to see you there!
- 1-3 Oct 2025 GAMRIC, the Global AMR Innovators Conference (London, UK). Formerly the ESCMID-ASM Joint Conference on Drug Development for AMR, this meeting series is being continued under the joint sponsorship of CARB-X, ESCMID, BEAM Alliance, GARDP, LifeArc, Boston University, and AMR.Solutions. The ongoing series will continue the successful format of prior meetings with a single-track meeting and substantial networking time (go here to see details of the outstanding 2024 meeting). Registration will open on 5 May 2025; in the interim, the preliminary agenda can be found at that same link (https://www.gamric.org/). The meeting will be limited to approximately 300 attendees, so please be sure to register promptly to avoid disappointment! The abstract submission window will run 5 May to 13 June and an application round for travel grants is expected to run in a similar time frame.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society of America. Details pending; go here for the general meeting website.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference. Sponsored by the BEAM Alliance, the 9th AMR Conference has just concluded and it’s again been an excellent meeting! Please mark your calendar for next year. You can’t register yet, but details will appear here!
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. You can’t register yet, but you can go here for details on the outstanding 2025 meeting.
Upcoming meetings of interest to the AMR community:
- [NEW] 21 May 2025 (6.30-9.00p CEST, in-person, Geneva): Sponsored by CGD (Center for Global Development), this seminar “From Evidence to Action: Designing an Effective Independent Panel on Antimicrobial Resistance” will feature the launch of new, interactive web tool that estimates the economic impact of AMR across 122 countries. Go here to register.
- 22 May 2025 (9.30-11.00a CEST, virtual): GARDP REVIVE webinar entitled “Post-licensing clinical trials for advancing the use of antimicrobials.” Go here for details and to register.
- 19-23 June 2025 (Los Angeles): ASM Microbe, the annual meeting of the American Society for Microbiology. Go here for details.
- 10-13 Sep 2026 (Lisbon, Portugal): 6th ESCMID Conference on Vaccines. Go here for details.
- 1-3 Oct 2025 GAMRIC, the Global AMR Innovators Conference (London, UK; formerly the ESCMID-ASM Joint Conference on Drug Development for AMR). See list of Top Recurring meetings, above..
- 11-19 Oct 2025 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2025 will be the 9th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications are being accepted from 20 Mar to 21 Jun 2025 — go here for more details.
- 17-20 Sep 2025 (Porto, PT): 14th International Meeting on Microbial Epidemiological Markers (IMMEM XIV). Go here for details.
- 9-13 Nov 2025 (Portland, OR, USA): ASM Conference on Biofilms. Go here for details and to register.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025. See list of Top Recurring meetings, above.
- 29-31 Oct 2025 (Bengalaru, India): ASM Global Research Symposium on the One Health Approach to Antimicrobial Resistance (AMR), hosted in partnership with the Centre for Infectious Disease Research (CIDR) at the Indian Institute of Science (IISc). Go here for details and to register.
- 28-30 Jan 2026 (Las Vegas, NV, USA): IDSA and ASM have announced a new US-based meeting series entitled IAMRI (Interdisciplinary Meeting on Antimicrobial Resistance and Innovation) and described as a “forum for collaboration and exploration around the latest advances in antimicrobial drug discovery and development.” You can’t register yet (further details anticipated June 2025) but you can go here to see general details about the new meeting.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference sponsored by the BEAM Alliance. See list of Top Recurring meetings, above.
- 8-13 Mar 2026 (Renaissance Tuscany Il Ciocco, Italy): 2026 Gordon Research Conference (GRC) entitled “Antibacterials of Tomorrow to Combat the Global Threat of Antimicrobial Resistance.” A Gordon Research Seminar (GRS) will be held the weekend before (7-8 Mar) for young doctoral and post-doctoral researchers. Space for the GRS and the GRC is limited; for details and to apply, go here for the GRC and here for the GRS.
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
Self-paced courses, online training materials, and other reference materials:
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.
- GARDP’s REVIVE website provides an encyclopedia covering a range of R&D terms, recordings of prior GARDP webinars, a variety of viewpoint articles, and more! Check it out!
- GARDP’s https://antibioticdb.com/ is an open-access database of antibacterial agents.
- The CARB-X website provides a range of recordings from its webinars, bootcamps, and more. A bit of browsing would be time well spent!
- British Society for Antimicrobial Chemotherapy offers an eLearning section: Education – The British Society for Antimicrobial Chemotherapy.