Dear All,
Today we have two interwoven topics to consider: An analysis of research priorities (not all technical!) and a triad of related initiatives, each of which comes with an opportunity for you to starting working on some of those priorities!
First up, we have an excellent new paper summarizing the perspective of 32 experts from 15 countries on what the global community needs to do both (i) to support development of new agents and (ii) to preserve the efficacy and maximize effectiveness of existing agents:
- Charani E, et al. Optimising antimicrobial use in humans; review of current evidence and an interdisciplinary consensus on key priorities for research. The Lancet Regional Health – Europe. https://doi.org/10.1016/j.lanepe.2021.100161
In brief, 4 areas for work are highlighted: policy and strategic planning; medicines management and prescribing systems; technology to optimise prescribing; and context, culture and behaviours. Based on these 4 pillars, the review lays out actions needed over the next 2-10 years:
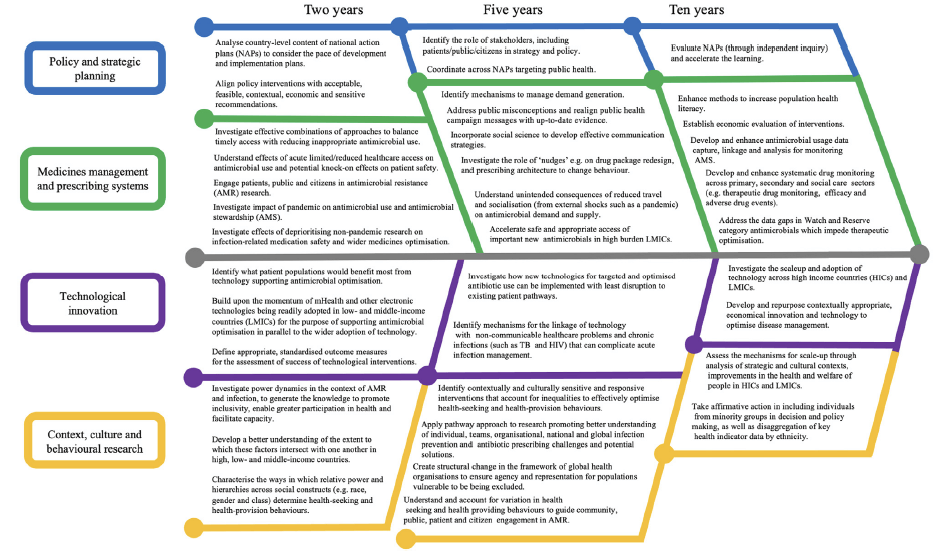
You’ll need to read the paper itself to get all the details, but one of the themes that emerges is that access depends both on harmonized regulatory pathways as well as the nuts-and-bolts of building and supporting high-quality health systems. So, and second on our tour today, we move to look at relevant work now being initiated by the AMR Industry Alliance (AMRIA).
Starting with its 2016 declaration released at Davos in which >100 companies and trade associations called for collective action to create a sustainable and predictable market for antibiotics, vaccines and diagnostics (23 Jan 2018 newsletter; the declaration, the roadmap), AMRIA has been pushing Industry to set norms for Industry contributions to the AMR problem by defining approaches to (i) appropriate use, (ii) access, (iii) manufacturing; and (iv) research and science.
With the hopeful signs that significant Pull incentives may come into being (22 Jun 2021 newsletter on PASTEUR in the US, 29 Mar 2020 newsletter on the UK NHS England pilot, 28 Nov 2020 newsletter on the EU Pharmaceutical Strategy and its call for Pull incentives, 29 Apr 2021 follow-up newsletter on the EU initiative), AMRIA is now engaging to address changes in the AMR ecosystem that are needed for all of these elements to work together harmoniously.
Specifically, AMRIA is now seeking consultants to help with 3 interesting projects (Melissa Gong Mitchell, AMR Industry Alliance Secretariat Lead, can be reached at mmitchell@amrindustryalliance.org with questions):
- Scaling Access in LMIC Hospital Settings (RFP): Develop a framework for appropriate access to diagnostics and antibiotics in hospital settings in LMICs and design a pilot initiative to test this framework. The deadline for this RFP is Friday 16 July 2021.
- AMR Innovation and R&D: Regulatory Landscape Phase 1 (RFP): Identify opportunities to foster regulatory alignment for innovation, clinical trials and commercialization through a landscape analysis. The deadline for this RFP is Friday 23 July 2021.
- AMR Innovation and R&D: Regulatory Landscape Phase 2 (RFP): Organize and facilitate a series of roundtable workshops to discuss findings from a commissioned landscape analysis report (see above) and develop a series of country roadmaps highlighting good practices in innovation, registration and commercialization. The deadline for this RFP is Friday 30 July 2021..
Even if these RFPs are not your cup of tea, it is very interesting to see the nature of this forward-thinking work. Many adjustments to the AMR ecosystem are needed and it’s great to see steps being taken to just get some momentum going:
- The RFP focused on access aligns with the goals of CARB-X’s requirement for a Stewardship & Access Plan (22 Mar 2021 newsletter).
- Note also the sequential and connected nature of RFP #2 and #3 on regulatory alignment: the idea of harmonized global handling of new antimicrobials is really very attractive — one of the reasons for lags in access to new antibiotics is the daunting challenge of the varied regulatory processes around the globe. FDA, EMA, and PMDA have been working for some years to harmonize their guidance (21 Nov 2017 newsletter on the FDA-EMA-PMDA conversations; 5 Jul 2020 newsletter on an FDA analysis of the potential for harmonized CABP endpoints) … and the work proposed by AMRIA would drive the conversations needed to make this a reality.
Is one of these opportunities for you? I hope so!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Current funding opportunities (most current list is here):
- CARB-X recently announced that their existing resources will be reserved to fund their existing portfolio (more than 80 total awards, and counting, as they include contracting from prior rounds). New rounds from CARB-X will occur only after new funding is obtained in 2021.
- It’s not a funder, but AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 6 Jul 2021 (virtual, 10-11.30 CET): South-East Asia WHO Regional and Country experiences in implementation of national action plans for AMR. WHO Webinar series, go here to register.
- 6 Jul 2021 (virtual, 14.00-15.00 CET): Eastern Mediterranean WHO Regional and Country experiences in implementation of national action plans for AMR. WHO Webinar series, go here to register.
- 9-12 Jul 2021 (virtual): Annual ECCMID meeting (#31)
- 14 Jul 2021 (virtual, 10.00-11.00 CET): The Role of Vaccines in Preventing Antimicrobial Resistance– WHO Perspectives and Country Experiences (Pakistan). WHO Webinar series, go here to register.
- 14 Jul 2021 (virtual, 16.00-17.00 CET): The Role of Vaccines in Preventing Antimicrobial Resistance– WHO Perspectives and Country Experiences (Zimbabwe). WHO Webinar series, go here to register.
- 20 Jul 2021 (virtual, 16.30-18.00 BST): BSAC-sponsored webinar entitled “Diagnostic-driven strategies for antimicrobial resistance in the UK” that will highlight AMR priorities and diagnostic needs for the UK’s NHS. Go here to register.
- 26 Jul-30 Jul 2021 (online): Small World Initiative Instructor Training Workshop – training for undergraduate professors in the wet lab techniques, parallel curricula, & pedagogical instruction to engage students in the hunt to find new antibiotic-producing soil microbes. Go here to register.
- 14-29 Aug 2021 (Marine Biology Laboratory, Woods Hole, MA): Residential course entitled “Molecular Mycology: Current Approaches to Fungal Pathogenesis.” This 2-week intensive training program has run annually for many years and gets outstanding reviews. Go here for details.
- 24-26 Aug 2021 (virtual, timings not stated but presumably EU-centered): The 5th edition of the annual AMR conference sponsored by the BEAM Alliance, CARB-X, the Novo REPAIR Impact Fund, the IMI Accelerator, and the European Biotechnology Network. The in-person version of this meeting is consistently excellent; the video-based version will have to do for 2021. Go here for details.
- 8-11 Oct 2021 (Aberdeen, Scotland): 10th Trends in Medical Mycology. Go here for details.
- 11-15 Oct 2021 (physical, somewhere in the UK): UK-focused Innovation Mission sponsored by Innovate UK in collaboration with AMR Insights and Oxford innovation. This free event seeks to connect AMR-focused start-ups, SMEs and Multinationals, Academia, Research Institutes, Regional Development Companies and other interested stakeholders in the UK, Europe and other parts of the world. Go here for more details.
- 16-24 Oct 2021 (Annecy, France): Interdisciplinary Course on Antibiotics and Resistance (ICARe). This is a soup-to-nuts residential course on antibiotics, antibiotic resistance, and antibiotic R&D. The course is very intense, very detailed, and gets rave reviews. Registration is here and is limited to 40 students. Bonus feature: For obvious reasons, the course didn’t happen in 2020! But as a celebration of the course’s 5th year, a webinar version was held on 29 Oct 2020: go here to stream it.
- 5-8 Nov 2021 (Albuquerque, New Mexico): Biannual meeting of the MSGERC (Mycoses Study Group Education and Research Consortium). Save-the-date announcement is here, details to follow.
- 6-11 Mar 2022 (Il Ciocco, Tuscany): Gordon Research Conference entitled “New Antibacterial Discovery and Development”. Go here for details, go here for the linked 5-6 Mar Gordon Research Seminar that precedes it.
- 25-28 Oct 2022 (Stellenbosch, South Africa): The University of Cape Town’s H3D Research Centre will celebrate its 10th anniversary with a symposium covering the Centre’s research on Malaria, TB, Neglected Tropical Diseases, and AMR. Go here to register.