Dear All,
In a thoughtful companion to WHO’s preclinical and clinical pipeline reviews, we now have an instructive analysis of the global funding pipeline for 2017-2023! Here are the links you need:
- The new paper and its primary data source:
- Paper: Czaplewski L, Lamichhane U, Sudbrak R, Hennessy A, Ogilvie LA, and Piddock LJV. An overview of global public and philanthropic investments into antibacterial therapeutics (2017-23). The Lancet Microbe. DOI: 10.1016/j.lanmic.2025.101288.
- Data: The Global AMR R&D Hub’s dynamic dashboard was the primary source for the new paper. It summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Useful related materials:
- 30 June 2020 newsletter: “FDA analysis of 40-years of antibacterial development: Dheman et al.” is well described by the newsletter’s title.
- WHO 2025 preclinical and clinical antibacterial pipeline review (data cut-off of 15 Feb 2025, published 2 oct 2024): Webpage about the report, the report itself, the initial AMR.Solutions newsletter about the report, and a follow-up newsletter about interactive dashboard versions of the report.
- WHO 2024 Bacterial Priority Pathogen List (PPL): Newsletter is here, report is here.
- The 4 Apr 2025 newsletter (“Antibacterial R&D is very hard! Two great pipeline reviews + an Industry-level view”) covers additional pipeline and timeline perspectives from 3 substantial reviews (Paterson 2024, Theuretzbacher et al. 2025, and IQVIA 2025).
By mining the data compiled over time by Global AMR R&D Hub, the authors were were able to show that US$ 2.5 billion has been invested in antibacterial R&D by 130 funders during 2017-2023. Funding peaked at $445m in 2020, declining then by 18% to $363m in 2023:
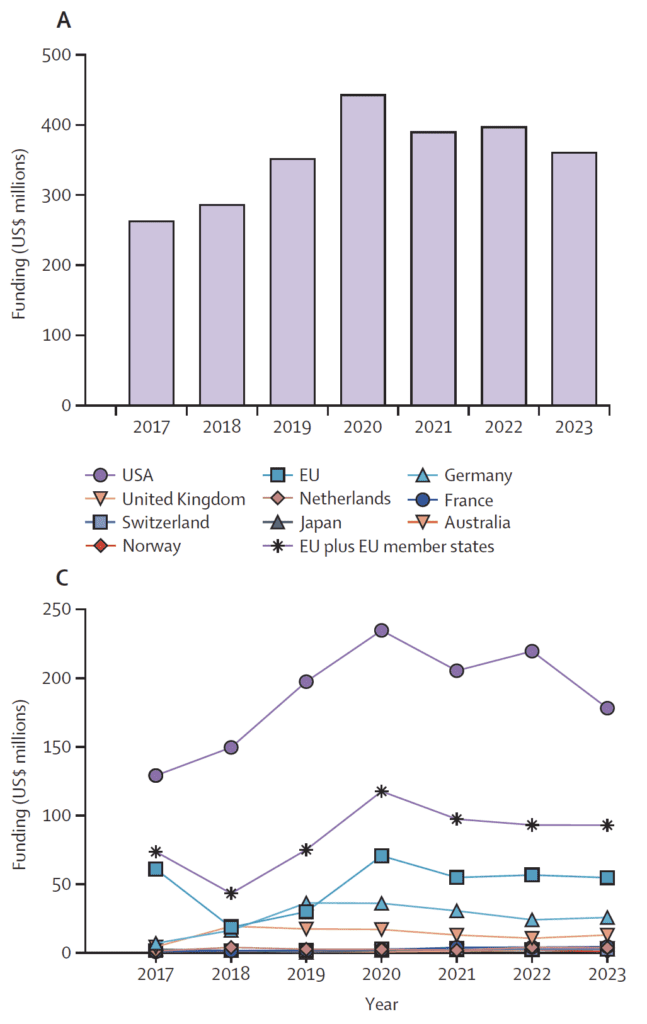
Above: Figure 1 from Czaplewski et al. The upper figure shows the total by year; the lower is the by-country/region breakout for those same years. Note that the EU appears both as itself alone (blue square) and in combination with local member state contributions (starburst).
It’s disturbing to see this fall-off in funding! The precise causes are not certain (COVID?), but the way that costs go up rapidly in later phases and FDA’s data [Dheman et al.] on how development timelines are getting longer certainly does not help (note that the y-axis is shown on a log10 scale):
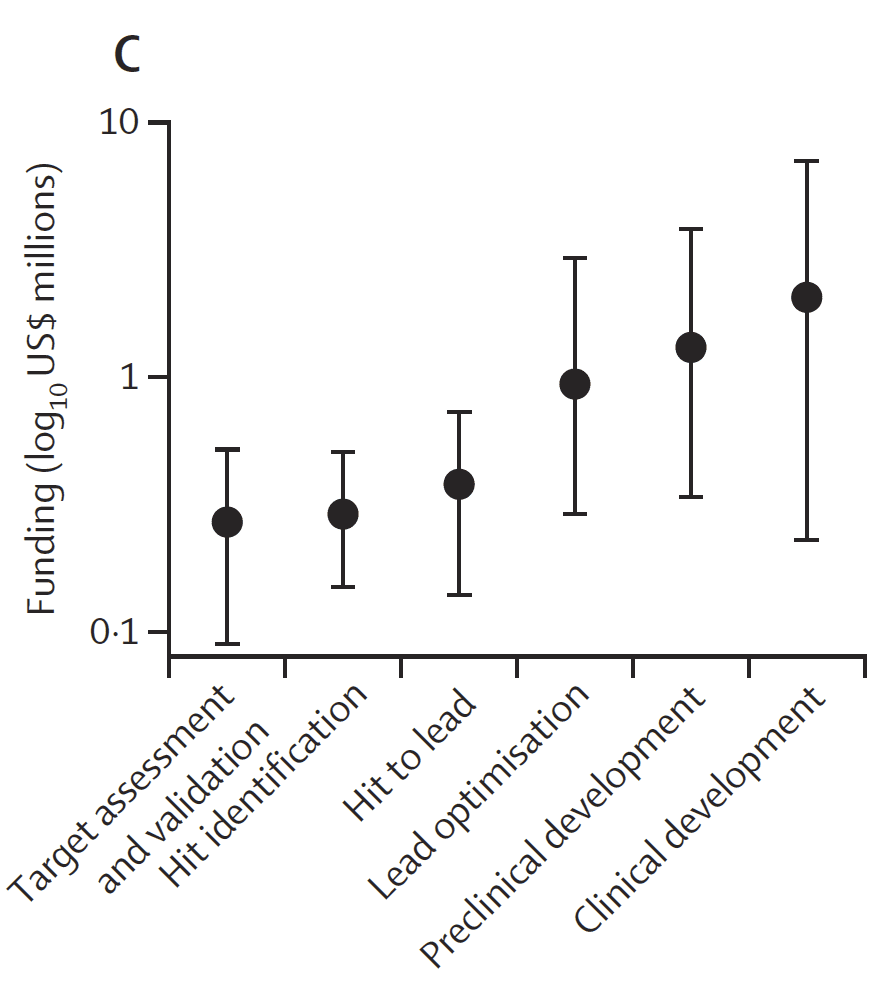
Above: Figure 4C from Czaplewski et al. showing total funding by stage from 2017 to 2023.
Other major observations include:
- Funding generally follows the WHO PPL list with 40% going to Gram-negative research, 18% to Gram-positive research, and 26% to work on M. tuberculosis.
- Traditional antimicrobials (that is, classical direct-acting products) are both better funded than non-traditional products and also have been more likely to make it into clinical development phases.
- For more on non-traditional products, see the 6 Aug 2019 newsletter (“Non-traditional antibiotics: A pipeline review and an analysis of key development challenges”). Yes, this newsletter is old but I’ve used it over time as a repository for updated materials … the most recent addendum is from 2026!
- 45% of the projects target single species! This one really surprised me … such products often sound attractive but are very dependent on ready availability of diagnostic tools and even then are surprisingly difficult to develop.
- For more on this, see the 1 Mar 2017 newsletter (“FDA Workshop: Animal models in support of narrow-spectrum agents for A. baumannii and P. aeruginosa“)
- The authors were encouraged by such recent developments as the Gram-Negative Antibiotic Discovery Innovator (Gr-ADI, 11 Feb 202t newsletter), a private sector commitment from GSK to GARDP, and the breadth of global investments into CARB-X.
- The authors conclude with clear calls for building sustained funding and ensuring its success by (i) focusing work on projects with clear translational pathways and (ii) building the workforce.
- I find the point about the workforce to be especially pressing. It takes a long time to gain knowledge in this space … and then people move into areas that offer better stability.
- See the 8 Feb 2024 newsletter entitled “Leaving the lab: The decline in AMR R&D professionals.”
Well done to the authors! Without funding there is no pipeline and I look forward to seeing this report reprised at intervals along with the product pipeline reviews.
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers, the small meeting format of BEAM’s AMR Conference (March) and GAMRIC (September-October; formerly, the ESCMID-ASM conference series) creates excellent global networking. IDWeek (October) and ECCMID (April) are much larger meetings but also provide opportunities for networking with a substantial, focused audience via their Pipeline sessions. Hope to see you there!
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference. Sponsored by the BEAM Alliance, the 9th AMR Conference was an excellent meeting! A draft program has been posted and registration is now open. Please plan to attend!
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. You can go here to register and view the preliminary program; the abstract submission window for 2026 will run 15 October to 26 Nov 2025. For those who would like a substantial opportunity to present a product to a large audience (see also adjacent note about IDWeek), I know that the meeting schedule will again include Pipeline Monday.
- 22-24 Sep 2026 (Lisbon, Portugal): The 2nd GAMRIC, the Global AMR Innovators Conference (London, UK). Formerly the ESCMID-ASM (or ASM-ESCMID depending on location) Joint Conference on Drug Development for AMR, 2026 will be the 11th year for this series that is now under the joint sponsorship of CARB-X, ESCMID, BEAM Alliance, GARDP, LifeArc, Boston University, and AMR.Solutions. The ongoing series employs the successful format of prior meetings with a single-track meeting and substantial networking time. The 2025 meeting was a sell-out success! A written summary of the meeting is here and the video from the sessions is now available here. Registration will open March 2026; the abstract submission window will be 10-31 March 2026.
- 21-24 Oct 2026 (Washington, DC, USA): IDWeek 2026, the annual meeting of the Infectious Diseases Society of America. Details are not yet available but I would expect the program to continue to provide a substantial opportunity to present a product to a large audience (see also adjacent note about ESCMID) as well as opportunities to present at an IDWeek Pipeline Session.
Upcoming meetings of interest to the AMR community:
- 18-20 Feb 2026 (Sydney, Australia, in person): The “AMR 2026 Summit”, hosted by the Fleming Initiative and Australia’s Science Agency, CSIRO. This event (website) will spotlight evidence-informed One Health approaches, practical solutions to implementation barriers, and strategies for public engagement, education, and advocacy. Space is limited, so (and sort of like applying to attend a Gordon Conference), please register your interest to attend here.
- 3-4 Mar 2026 (Basel, Switzerland): The 10th AMR Conference sponsored by the BEAM Alliance. See list of Top Recurring meetings, above.
- 8-13 Mar 2026 (Renaissance Tuscany Il Ciocco, Italy): 2026 Gordon Research Conference (GRC) entitled “Antibacterials of Tomorrow to Combat the Global Threat of Antimicrobial Resistance.” A Gordon Research Seminar (GRS) will be held the weekend before (7-8 Mar) for young doctoral and post-doctoral researchers. Space for the GRS and the GRC is limited; for details and to apply, go here for the GRC and here for the GRS.
- 17-21 April 2026 (Munich, Germany): ESCMID Global 2026, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 4-8 June 2026 (Washington, DC): ASM Microbe, the annual meeting of the American Society for Microbiology. The meeting format is evolving and next year will combine 3 meetings (ASM Health, ASM Applied and Environmental Microbiology, and ASM Mechanism Discovery) into one event. Go here for details.
- 22-24 Sep 2026 GAMRIC (Lisbon, Portugal), the Global AMR Innovators Conference (London, UK; formerly the ESCMID-ASM Joint Conference on Drug Development for AMR). See list of Top Recurring meetings, above..
- 10-18 Oct 2026 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance) … and 2026 will be the 10th year for this program. Patrice Courvalin orchestrates content with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Registration for 2026 will not open for some time; go here for more details and put a reminder in your calendar to check back in the Spring if you are interested.
- 21-24 Oct 2026 (Washington, DC, USA): IDWeek 2026. See list of Top Recurring meetings, above.
- 10-13 November 2026 (Madrid, Spain): The International Society for Infectious Diseases (ISID) has announced its 21st International Congress on Infectious Diseases (ICID). Register and view the preliminary program here. Note as well that the organizers have an open call for topic proposals with a 20 Jan 2026 deadline.
Self-paced courses, online training materials, and other reference materials:
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.
- GARDP’s REVIVE website provides an encyclopedia covering a range of R&D terms, recordings of prior GARDP webinars, a variety of viewpoint articles, and more! Check it out!
- GARDP’s https://antibioticdb.com/ is an open-access database of antibacterial agents.
- The CARB-X website provides a range of recordings from its webinars, bootcamps, and more. A bit of browsing would be time well spent!
- British Society for Antimicrobial Chemotherapy offers an eLearning section: Education – The British Society for Antimicrobial Chemotherapy.
- [NEW] FDA have issued two AMR-related RFPs: One for interpretive criteria for in vitro activity vs. Candida auris and one for ideas on using large public datasets to generate control data for invasive fungal infections. The submission deadline for both is 24 Feb 2026. See the 5 Feb 2026 newsletter for details (“FDA RFPs: Susceptibility breakpoints and external controls for invasive fungal infections”).
- The Horizon Europe Work Programme 2026-2027 includes at least 3 calls of interest within its Cluster 1 — see the list below. The application window starts 10 Feb 2026 and closes on 16 Apr 2026. See also the 12 Dec 2025 newsletter about the call. Note as well that there calls for agents to prevent and/or treat viral infections.
- HORIZON-HLTH-2027-01-DISEASE-08: Development of innovative antimicrobials against pathogens resistant to antimicrobials
- HORIZON-HLTH-2027-02-IND-02: Portable point-of-care diagnostics
- HORIZON-HLTH-2026-01-DISEASE-03:Advancing research on the prevention, diagnosis, and management of post-infection long-term conditions.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- CARB-X will have calls during April 2026 and 4Q 2026. The team have announced that at least one of the themes of both calls will be novel chemistry scaffolds.
- These things aren’t sources of funds but would help you develop funding applications
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Antimicrobial Resistance Research and Innovation in Australia is an actively updated summary that covers Australia’s AMR research and patent landscape. It is provided via collaboration between The Lens (an ambitious project seeking to discover, analyse, and map global innovation knowledge) and CSIRO (Commonwealth Scientific and Industrial Research Organisation, an Australian Government agency responsible for scientific research). Lots to explore here!
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).