Dear All (and with thanks to Kevin for co-authoring),
The UK NHS England Pilot is known to us all as the first real attempt at an antibiotic subscription model and now it will be undergoing a major overhaul. In case you are a new reader or would like a refresher on the pilot, here are the links you will need:
- 10 Jul 2023 announcement of the consultation on the updated program
- The proposal for consultation and the product evaluation criteria
- “Incentivising New Antibiotics: Designing a Value-Based Delinked Pull Incentive Mechanism” report from the UK’s Office for Health Economics
- 10 April 2023 newsletter analyzing the report
- 11 April 2022 newsletter covering the data behind the value of two initially selected antibiotics.
- 29 March 2020 newsletter discussing the original approach to the pilot scheme
- 29 November 2019 newsletter with details of the original plans of the pilot.
- For more economics-related details: 6 March 2020 newsletter with related discussions of economics and 29 November 2022 newsletter on the ROI of antibiotics.
- Prefer a video? Watch the NHS Pilot video and the “STEDI” video from the YouTube channel.
Two drugs were chosen last year to spearhead the subscription model but, during the process, it was clear the initial parameters of the program were too limited. In this latest update, the pilot plans to expand in a huge way:
- While the name ‘UK Pilot’ may seem to imply the entire country, the program was only run in England. Now that it has proven to be a success, it will expand from just England to the whole of the United Kingdom.
- The evaluation panel’s approach to determining the value of contract payments will be updated to reflect what was learned in assessing the first two products.
- With this new expansion, the contracts offered could be doubled from £10 million/year to £20 million/year for qualifying products that match the standards set by the evaluation panel. Products that combat pathogens that are high on the WHO Priority Pathogen List are of particular interest!
The big step forward is that instead of repeating the pilot’s approach of applying quantitative health economic analyses on a product-by-product basis, the evaluation panel will instead use a points-based scoring system that is rooted in the learnings from the pilot. The UK team highlight that an important conclusion in support of this approach is that the two products in the pilot were shown to have sufficient value to justify paying England’s ‘fair share’ towards antimicrobial research and development.
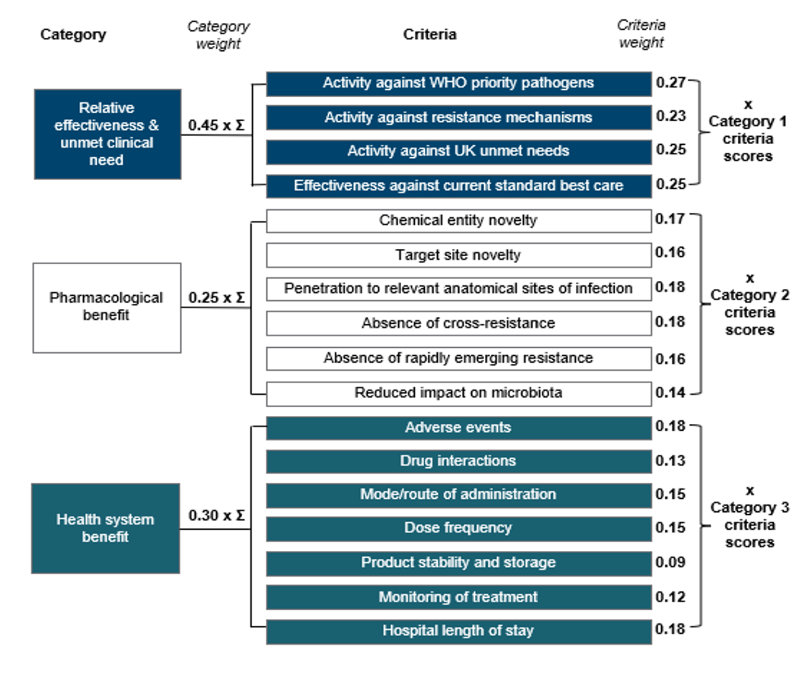
The new scoring system does this with a transparent multi-criteria decision analysis tool built on 3 categories of value: (1) Relative effectiveness & unmet medical need; (2) Pharmacological benefit; and (3) Health system benefit, Collectively, these categories capture some of the STEDI values that are overlooked by traditional HTAs, but which were captured in the pilot.
The structure of the scheme can be seen in the image below. It’s really instructive to see how the relative weight of different factors has evolved since the pilot — pathogen spectrum, degree of novelty, and ease of use all have a role. Everyone sees the categories, weights and scoring system. With this, a developer can make development trade-offs knowing their consequences — a team can internally score their products to get a sense as to whether they would qualify for a UK subscription.
Next steps and a call for your feedback: A 12-week public consultation period is launching on 10 July 2023 and is seeking input from experts across the board. If you would like to share your opinions, please do so via this link. Now is the time to engage. Make it so!
—
[Alert: Increasingly wonkish text from this point!] Now that we’ve covered the basics of this big news, let’s do a dive into what this could look like in reality. Rewarding high-value antibiotics is a difficult and delicate process starting with defining what is high-value. We are all familiar with doing this in other settings — as one well-known example, think of the way NIH funds research via a peer-reviewed scoring system. No two organizations have the same standards or approval process and that is not a bad thing. The more trial and error in tandem with sharing information with our peers, the better … this is how the overall ecosystem is refined.
So, how do we do this for new antibiotics? Well, it turns out that a lot of work has gone into this over the past 10 years. No one wants to sponsor a mediocre product! As that recent JAMRAI multi-country survey drove home, governments want to create a Pull incentive that will be focused on high-impact products. The desire to avoid paying for a lemon is really important in this debate: don’t miss Christine Ardal’s comments on this — you can get it in part from the newsletter but you can also jump to 11:18 in the video and hear it straight from her.
Based on these themes, our goal should be to set high but attainable standards. If we clearly set these standards now, R&D teams can plan for them as projects evolve. And, we want the standards to be relatively similar on a global basis so that project teams can know that they are moving in a generally acceptable direction. On this point, the idea of “Shared Valuation Principles” was called out at the 4 June 2021 meeting of the G7 Health Ministers (see this 5 June 2021 newsletter and its YouTube video explainer for more).
To make this work, we need to do two things in parallel – measure the financial value to society (why is this worth paying for?) and develop scoring systems for measuring new products (what do we pay for?). To that end, you will find annotated reading lists for both themes below the signature block. The papers are given in chronological order … as you review them, you’ll readily see how elements have emerged that are now captured in the NHS scoring scheme.
In summary: The NHS Pilot’s initial scheme did more than just create a plan for the UK — it made the UK a world leader in developing an antibiotic subscription plan. It was a bold step and, after hearing the criticism from the original standards, we now are set to see further refinements to their scheme following the results of this consultation period.
Thunderous round of applause for Team UK! What a year this has been thus far! Now, get to it and get involved with the UK’s public consultation.
All best wishes, John & Kevin
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
Kevin Outterson, JD, Professor of Law, Boston University & Executive Director, CARB-X (these views are personal and do not necessarily reflect the views of CARB-X or any of its funders) @koutterson
Financial Value Metrics (for more, see the Incentives page of AMR.Solutions website)
- 2014: Analytical Framework for Examining the Value of Antibacterial Products from US Assistant Secretary for Planning and Evaluation
- This was one of the first serious efforts to estimate the societal value of an antibiotic. The gap between the commercial value of a new drug for HABP/VABP and its value to society was staggering: -$4.5 million vs. $1.2 billion. Yes, that’s a negative sign in front of the $4.5m … not a typo.
- 2014-16: Review on Antimicrobial Resistance led by Lord Jim O’Neill (aka the O’Neill Report)
- Without action to create new antibiotics, cumulative global GDP losses as high as $100 trillion by 2050 along with as many deaths as cancer were estimated as possible.
- 2016: Drug-Resistant Infections: A Threat to Our Economic Future from The World Bank
- Without action to create new antibiotics, the World Bank estimated that pandemic spread of AMR pathogens could lead to a global loss of annual GDP in the 1-4% range, an impact as large as the losses provoked by the 2008–2009 global financial crisis.
- 2018: Framework for value assessment of new antimicrobials: implications of alternative funding arrangements for NICE Appraisal from the University of York
- The STEDI principles for assessing value are identified (Spectrum, Transmission, Enablement, Diversity, and Insurance; see this newsletter, this newsletter, and this YouTube explainer for more on STEDI).
- See also this webpage from the same group in which they provide the assessments that underpin the first two drugs in the pilot: Assessing the value of novel antimicrobials under new payment models
- 2018: Stemming the Superbug Tide — Just A Few Dollars More by the OECD
- This report concludes that “Every year between 2015 and 2050, up to USD 3.5 billion (adjusted for differences in prices across countries, expressed as purchasing power parities or PPPs) is expected to be spent on average between 2015-2050 on AMR-related complications across 33 OECD and EU28 countries, according to calculations from the OECD model.”
- The underlying model is being updated by the recently formed Quadripartite Technical Group on the Economics of Antimicrobial Resistance (QTG-EA) as part of the run-up to UNGA 2024.
- 2022: Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis by Murray et al.
- This important paper looks at the human cost and concludes that “On the basis of our predictive statistical models, there were an estimated 4·95 million (3·62–6·57) deaths associated with bacterial AMR in 2019, including 1·27 million (95% UI 0·911–1·71) deaths attributable to bacterial AMR.”
- 2022: An Ambitious USG Advanced Commitment for Subscription-Based Purchasing of Novel Antimicrobials and Its Expected Return on Investment and The World Needs New Antibiotics. A Proposed US Program to Develop Them Would Pay Off 28:1 from the Center for Global Development
- As another variation on the human cost, this paper looks at the $ value in terms of DALYs (Disability-Adjusted Life Years) and concludes that $4.9T would be saved by spending $40B on new antibiotics (note that it is 4.9 Trillion vs. 40 Billion!)
Integrated Scoring Schemes
- 2013: The US’s GAIN Act proposed the broad concept of Qualified Infectious Diseases Product (QIDP)
- QIDP was based entirely on spectrum and this meant that almost everything qualified. We now recognize that this is too broad. Thus additional element are needed to define value. Read on…
- 2016: Antibiotic reimbursement in a model delinked from sales: a benchmark-based worldwide approach
- We (Outterson and Rex) defined a system in which spectrum, mechanistic novelty, and breadth of use (oral dosing, pediatric data) were proposed as ways to define value
- WHO’s scoring scheme for products in development
- Theuretzbacher et al. (2020) Critical analysis of antibacterial agents in clinical development
- WHO (2021) Antibacterial agents in clinical and preclinical development: an overview and analysis
- Butler et al. (2022): Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed
- This was the big step forward! The scoring tool used here both considered pathogen spectrum and proposed ways to value lack of cross-resistance, chemical novelty, target-based novelty, and mechanistic novelty … you’ll see that these appear in the NHS scoring scheme
- 2023-2024: PASTEUR Act
- Doesn’t define a scheme but clearly creates a path to one!
Current funding opportunities (most current list is here)
- The AMR Industry Alliance has announced that applications are again open for its annual Stewardship Prize. The Alliance began the Stewardship Prize in 2021 to identify and support innovative approaches to combatting antimicrobial resistance in low-to-moderate-income countries — the winning application receives CHF 10,000. Applications close September 1, 2023; go here to see past winners and here to apply.
- The AMR Action Fund is now open to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- BARDA’s long-running BAA-18-100-SOL-00003 offers support for both antibacterial and antifungal agents. This BAA has offered 4 deadlines/year since 2018 … check the most current amendment for details.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
Upcoming meetings of interest to the AMR community (most current list is here):
- 3-5 Jul 2023 (Tours, France): 9th Symposium on Antimicrobial Resistance in Animals and the Environment (ARAE). Sponsored by INRAE (French National Research Institute for Agriculture, Food, and Environment, itself a merger of merger of INRA, the French National Institute for Agricultural Research, and IRSTEA, the French National Research Institute of Science and Technology for the Environment and Agriculture), this conference has been running since 2005. Go here for details.
- 4-6 Aug 2023 (Bangkok, Thailand): The regional Medical Mycology Training Network Conference by ISHAM’s Asia Fungal Working Group is set to feature both hands-on workshops and clinical sessions for all those managing and working with invasive fungal infections. Go here for details.
- 19-22 Sep 2023 (Boston, USA): ASM-ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. This is an excellent development focused meeting … highly recommended! Go here for details and to register.
- 7-15 Oct 2023 (residential, Annecy, France): ICARe, the Interdisciplinary Course on Antibiotics and Resistance. Now in its 7th year, this course is a deep-dive into the world of antibiotic development. Intense, rigorous, and HIGHLY recommended. Seats are always limited … apply sooner rather than later! Go here for details.
- 11-15 Oct 2023 (Boston, USA): IDWeek 2023, the annual meeting of the Infectious Diseases Society of America. Go here for details and to register.
- 20-23 Oct 2023 (Athens, Greece): 11th TIMM (Trends in Medical Mycology). Go here for details.
- 6-7 Feb 2024 (online): Antimicrobial Chemotherapy Conference. This is an annual, free of charge conference that is co-organized by GARDP and the British Society for Antimicrobial Chemotherapy (BSAC). Details to follow — for now, just mark your calendar.
- 27-30 April 2024 (Barcelona, Spain): 34th ECCMID, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.